Diagnostics of oral mucosae
A wide variety of benign lesions and diseases are detected on the oral mucosa. Oral mucosal lesions can also be associated with an underlying systemic disease. The correct diagnosis of mucosal lesions, which may share similar clinical and demographic features, is a challenge for a dentist and general practitioner. Diagnostics of oral mucosal lesions is based on a thorough investigation of the patient and a careful anamnesis. In addition, diagnostic tests, including biopsies and microbiologic samples, are usually required for setting a proper diagnosis. This is particularly important for early detection of premalignant lesions and oral cancer, because their prognosis is mainly dependent on the stage of the disease at the time of diagnosis. Since bacteria, fungi, and viruses are causative agents in a number of mucosal lesions and diseases, microbiologic samples are needed, if any infectious etiology is suspected. Blood tests are often helpful for diagnosis of systemic diseases.
Headlines | |
|---|---|
|
Mucosal lesions and diseases with different etiologies may share similar clinical features. Tissue biopsies, microbiological samples, and blood tests are often required for setting the correct diagnosis. |
Oral mucosa lines the oral cavity and protects the body from debris and infective agents. More than 200 diseases can be found on the oral mucosa. Mucosal lesions (abnormality of the mucosa) can be caused by a disease or local factors such as trauma or mechanical irritation. Mucosal lesions may also develop as a lack of some nutrients. Many oral lesions are asymptomatic and sometimes also difficult to recognize. Identification of oral mucosal lesions is an essential part of the oral health care. Patients with oral mucosal lesions can be diagnosed and treated in general dental practice but they may also be referred to a specialist in oral medicine, pathology or surgery, dermatologist or internal medicine. Oral surgery and medicine units of regional/university hospitals diagnose and treat patients with oral mucosal disease.
Oral mucosa
Oral mucosa is divided into three subtypes: keratinized masticatory (gingiva and hard palate), non-keratinized lining (buccal mucosa, floor of the mouth, ventral surface of the tongue, intra-oral surfaces of lips, soft palate), and specialized mucosa in the area of taste buds on lingual papillae (dorsal surface of the tongue). It is composed of stratified squamous epithelium and lamina propria, including connective tissue that contains blood vessels and lymph vessels, nerves, capillaries, and minor salivary glands. Oral epithelium is demarcated from connective tissue by a thin sheet of highly specialized extracellular matrix called basement membrane. A high rate of cellular turnover is characteristic of the oral epithelium. Thus, the healing capacity of the oral mucosa is greater than that of the skin. The major secretion associated with the oral mucosa is saliva, produced by major and minor salivary glands. Variations of normal mucosal anatomy, including Fordyce´s granules, fissured tongue, geographic tongue, and leukoedema, are rather common.
Resident microbiota of the oral cavity
A wide variety of different bacteria (700 up to 1,000 bacterial species and not-yet-cultivated phylotypes) have been found as colonizers of the human oral cavity (1, 2). It has been estimated that more than half of them still remain uncultivated. Most of the oral species/phylotypes have been detected in subgingival biofilms (1), while fewer findings come from mucosal surfaces where biofilm formation, due to constant epithelial shedding, is less pronounced.
Bacteria inhabit steadily the oral mucosae after the birth, when primary colonizers are viridans streptococci, Streptococcus mitis in particular, but also obligate anaerobes, representing the genera Veillonella, Prevotella, and Fusobacterium, appear before the eruption of the first tooth (3). The success of S. mitis in comprehensively colonizing oral mucosal surfaces, despite of the presence of secretory immunoglobulin A (IgA) in saliva, could be explained by its production of IgA1 protease. Once the colonization has occurred, bacterial species tend to persist in the oral cavity. However, at clonal level, intensive strain turnover among resident species, such as S. mitis and Fusobacterium nucleatum, is common (4, 5). The versatility of the oral microbiota increases considerably during the early years of life (3) but, not until the late adolescence, the composition resembles that of adults (6). Although enteric/environmental rods in the mouth are mainly associated with oral infections in immunocompromised subjects, they are rather common in infants and children but with decreasing prevalences due to increasing age (3, 6). Staphylococci as well are frequent findings in early months of life (3). As assessed with advanced molecular methods, the major genera present in saliva of children with a deciduous or mixed dentition and adolescents with a permanent dentition are Streptococcus, Veillonella, and Prevotella (6).
Although there is a «core oral microbiome» with hundreds of distinct species shared by healthy individuals (7), the bacterial composition varies considerably between individuals (8), probably due to differences, for example, in diet and health behavior (e.g., oral hygiene, smoking). While many bacterial genera are common to all sites of the mouth (8), different anatomical sites harbor a unique microbiota at a species level (1, 9, 10); however, some species, including S. mitis, Granulicatella adiacens, and Gemella haemolysans, colonize more or less all oral surfaces (9). It has been estimated that one individual usually harbors 30 - 70 species in the mouth and on each mucosal (cheek, dorsum of the tongue, lateral tongue, vestibule, hard palate, soft palate, labial gingiva) and tooth (supragingival, subgingival) surface 20 - 30 species (9). In general, there is homeostasis within these oral bacterial communities, i.e., their compositions stay relatively stable over time (11).
Due to aging, factors with potential impact on the oral microbiota include various long-term medications with salivary flow reduction, impairment of cognitive and/or motor skills to maintain good oral hygiene, and wearing dentures due to tooth loss (11). Despite of these kinds of factors, Streptococcus, Veillonella, and Fusobacterium, notably same genera as found in children, dominate in the oral cavity in elderly (12). Especially the dorsum of the tongue in subjects aged between 73 - 93 years and having a relatively good oral health status proved to have a rich microbiota, distinct from other oral sites, while recoveries from cheek and hard palate surfaces had closely related bacterial profiles and highest diversities (12). Also Pseudomonas was among the recoveries from buccal fold and the hard palate. The altered immune response due to aging may result in a higher bacterial diversity compared to younger adults (11). In edentulous subjects with complete dentures, three types of bacterial clusters can be recovered from oral surfaces: bacteria from the dorsal and lateral tongue as well as saliva formed one cluster and those from other mucosal surfaces another cluster, while bacteria from hard, inert denture surfaces formed the third cluster (13). The highest DNA probe counts were detected on the dorsum of the tongue and attached gingiva, followed by the exterior, polished denture surface, and the lowest counts on the hard palate.
Candida and other fungal species are frequent colonizers of the healthy oral cavity, where they interact with the bacterial microbiota (14). A considerable proportion of healthy individuals can be colonized with fungal species, such as Candida (75 %), Clodosporium (65 %), Aureobasidium (50 %), and Saccharomycetales (50 %), and up to 100 different fungi was recovered from 20 individuals with a healthy mouth (15). The interplay between oral bacteria and fungi may be seen beneficial in maintaining the health in the oral ecosystem (14).
Not only bacteria and fungi, but also Archaea, protozoa, and viruses are detected in the human mouth (2). All oral microbes may be seen as members of the resident oral microbiota in healthy carriers. In cases of ecologic disturbance, however, they can behave as opportunistic pathogens.
Mucosal lesions
The prevalence of oral mucosal lesions varies between 6 - 62 % (see Table 1). The detection rates vary due to differences in the methodology of recording mucosal lesions; for instance, some studies have included variations of normal anatomy in mucosal pathology. The majority of lesions are non-neoplastic and related to local irritation or trauma, such as habitual biting or denture rubbing. The most common mucosal anatomic variations/lesions detected are Fordyce´s granules, fissured tongue (Fig.1), geographic tongue, ulcers, pigmented lesions, and focal (frictional) hyperkeratosis (Table 1). A higher prevalence of oral mucosal lesions occurs among elderly populations (21, 24). Elderly have often a denture-related mucosal lesion, like angular cheilitis, traumatic ulcers, or denture stomatitis (24, 26). A higher percentage of smokers have oral lesions compared to non-smokers (20, 25). For example, the smoker´s palate is a common tobacco-related lesion that presents white keratinization of palatal mucosa with red dots, representing an inflamed salivary duct orifice.
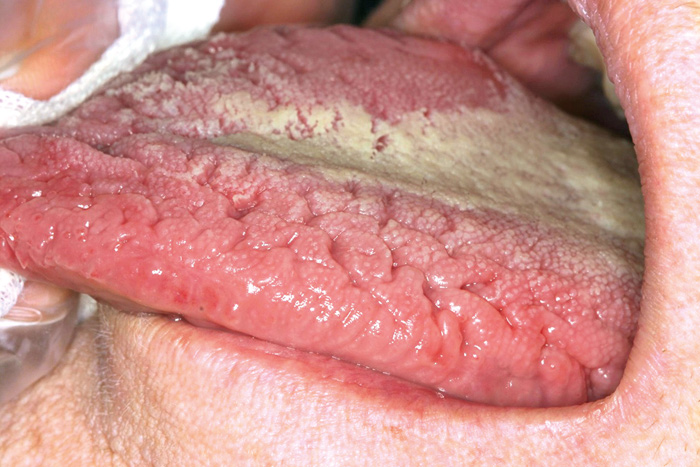
Fig. 1. Fissured tongue.
Age group (years) |
Study group (n) |
Prevalence (%) |
Most common lesions |
Study |
5 - 95 |
765 |
42 |
Excessive melanin pigmentation, fissured tongue, denture stomatitis |
Mumcu et al. 2005 (16) |
-15 |
18659 |
Leukoedema, geographic tongue, lichen planus |
Axéll 1976 (17) |
|
17 - 85 |
5000 |
16 |
Aphtous ulcers, coated tongue, secondary herpes |
Cebeci et al. 2009 (18) |
17 - 29 |
all 17235 |
19 |
In the whole study group: denture-related lesions, tobacco-related lesions, amalgam tattoos, cheek/lip bites, frictional keratosis |
Shulman et al. 2004 |
30 - 39 |
23 |
(19) |
||
40 - 49 |
29 |
|||
50 - 59 |
36 |
|||
60 - 69 |
39 |
|||
-70 |
43 |
|||
-20 |
106 |
26 |
In the whole study group: white, red, pigmented lesions |
Ali et al. 2013 (20) |
21 - 40 |
207 |
71 |
||
-41 |
217 |
62 |
||
20 - 29 |
all 6267 |
6 |
In the whole study group: exophytic neoplasia, leukoplakia simplex |
Splieth et al. 2007 (21) |
70 - 81 |
20 |
|||
25 - 75 |
1609 |
62 |
Fordyce´s condition, fissured tongue, varices |
Kovac-Kovacic and Skaleric 2000 (22) |
-19 |
66 |
26 |
In the whole study group: Fordyce´s granules, fissured tongue, leukoedema |
Jahanbani et al. 2009(23) |
20 - 29 |
165 |
35 |
||
30 - 39 |
123 |
54 |
||
40 - 49 |
97 |
55 |
||
50 - 59 |
73 |
64 |
||
60- |
74 |
74 |
||
35 - 44 |
655 |
66 |
History of labial herpes and aphtous ulcers, Fordyce´s granules |
Reichart 2000 (24) |
65 - 74 |
1367 |
66 |
Fordyce´s granules, history of labial herpes, plicated tongue, denture stomatitis |
|
-40 |
1004 |
15 |
Recurrent aphtous ulcerations |
Pentenero et al. 2008(25) |
40 - 60 |
1939 |
25 |
Frictional lesion |
|
60- |
1155 |
35 |
Denture stomatitis |
Soft tissue enlargements of oral mucosa comprise a diverse number of entities, ranging from reactive lesions to malignant tumors. In case of swellings, tissue biopsy is needed to result in a deductive diagnosis. The most common soft tissue lesions are reactive mucosal hyperplasia, mucoceles, and pyogenic granulomas (Fig.2) (17, 21, 27).
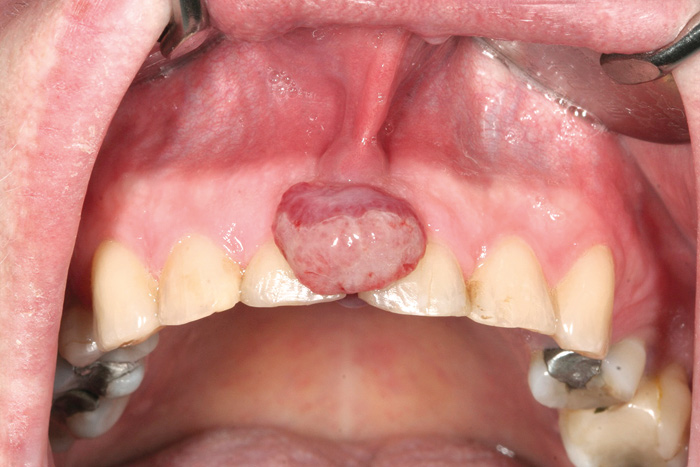
Fig. 2. Pyogenic granuloma.
Benign vascular lesions, both malformative, reactive, and neoplastic, are also common in oral soft tissues. Pigment lesions are usually amalgam pigmentations but also due to a melanotic macule, melanocytic nevus or the use of certain medication or oral hygiene products. Multiple melanotic macules can be associated with systemic conditions like Addison´s disease or Peutz-Jegher´s syndrome. In case of mucosal pigment lesions, the possibility of oral melanoma has to be taken into account, even though it is very uncommon.
Oral ulcers
Different types of ulcers are common on oral mucosae. Oral ulcers and erosions are associated with a wide variety of factors, ranging from vitamin deficiency to significant pathology (Figures 3, 4 and 5). An important feature is whether there is one ulcer or multiple ulcers. Malignant tumor usually appears as a single lesion and that is why a single ulcer with no signs of obvious healing within 3 weeks must be excised for a histological examination. In general, a granular ulcer with fissuring or raised exophytic borders may indicate malignancy, but the clinical picture of oral cancer can also be very variable (see Figure 3A). A single ulcer is commonly caused by trauma or aphthae, usually in persons who are otherwise well. Traumatic ulcers are often related to sharp teeth or restorations and in denture-wearers to resorbed residual alveolar ridge and lack of denture stability. Several systemic diseases may cause persistent single or multiple oral ulcers, and they are discussed further (see Table 2, Fig. 6).
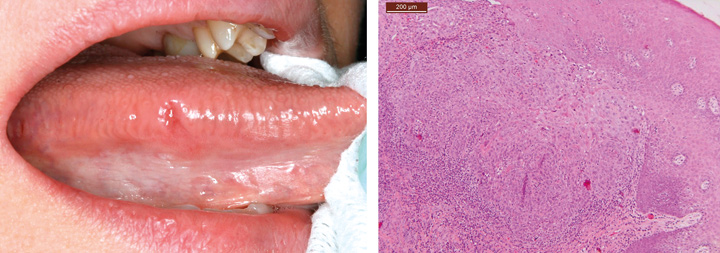
Fig. 3. Clinical picture of an oral mucosal lesion does not always tell the true nature of the lesion. A. A 49-year-old woman had persistent small ulcerative lesion on the border of the tongue. B. Histologically, the lesion turned out to be squamous cell carcinoma.
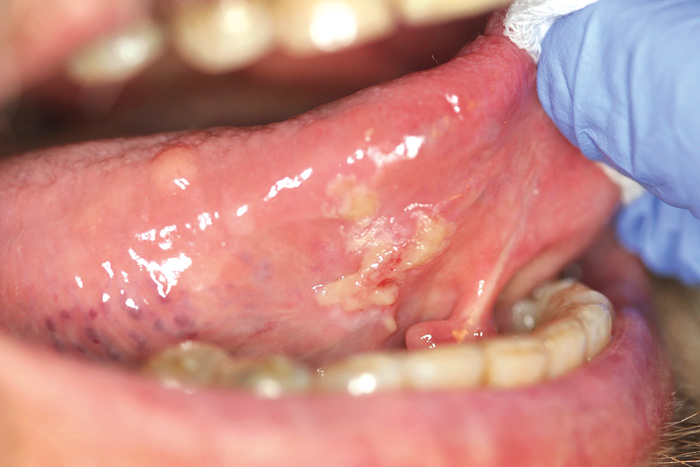
Fig. 4. A 72-year-old man with diabetes and rheumatoid arthritis had a pseudomembranous ulcerative lesion on the bottom of the tongue. Tissue biopsy revealed HSV-1 infection combined with secondary Candida infection (the lesion healed totally after topical medication).
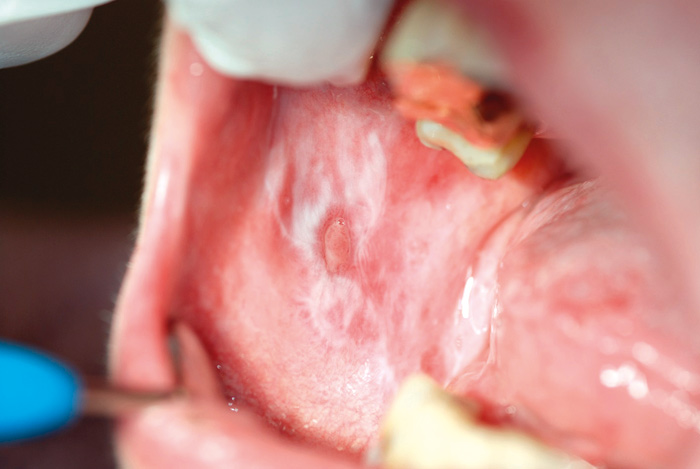
Fig. 5. Ulcerative lichen lesion on the buccal mucosa of a 63-year-old woman.
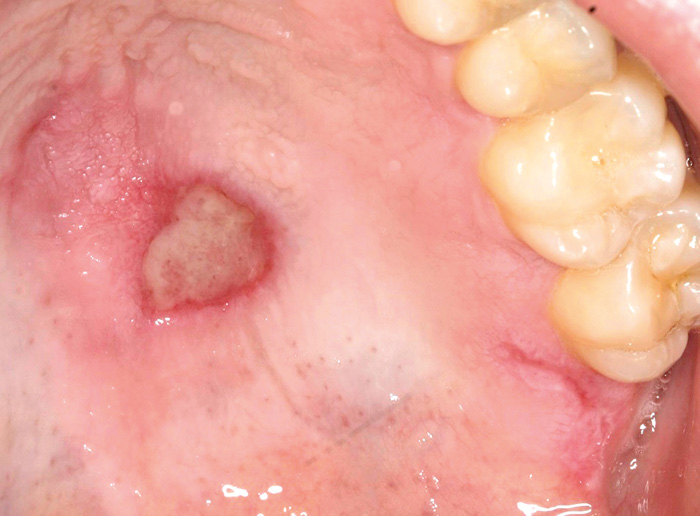
Fig. 6. A 30-year-old male with systemic lupus erythematosus presented a palatal ulcer typical for lupus. An incisional biopsy was performed to confirm the benign nature of the lesion.
Disease entity |
Diagnosis |
Oral mucosal lesion |
Skin diseases |
Lichen planusErythema multiformePemphigoidPemphigusEpidermolysis bullosaDermatitis herpetiformis |
White striae, papules, plaque, red atrophic areas, ulcers, bullaeWidespread blisters and ulceration, lip swelling and crustingBullae or vesicles, erosions and ulcers after the blisters burst, desquamative gingivitisVesiculobullous, erosionsBlistering after traumaVesicles, desquamative gingivitis |
Diseases of the gastrointestinal tract |
Celiac diseaseCrohn´s diseaseUlcerative colitisRefluxAnorexia, bulimia and other eating disorders |
UlcersUlcers, cobblestone lesions, reddish raised gingival lesions, lip swellingUlcersUlcersDryness |
Blood diseases |
AnemiaThrombocytopeniaNeutropeniaLeukemia |
Atrophy, atrophic glossitis, angular cheilitisUlcersUlcersPetechial hemorrhages, gingival bleeding |
Autoimmune conditions |
Sjögren syndromeSystemic lupus erythematosusDiscoid lupus erythematosusSclerodermaSarcoidosis |
Dryness, red and wrinkled mucosa, atrophy of tongue papillaeUlcers, erythema, hyperkeratosisReddish, ulcerated areas surrounded by white radiating striaeConstriction of the mouth, xerostomia, smooth appearance of the tongueUlcers, nodules -sometimes granularity, hyperkeratotic |
Vasculitis |
Granulomatosis with polyangiitis |
Granular, reddish gingival hyperplasia |
Endocrine disorders |
Diabetes mellitusAddison´s disease |
Dry, atrophy of the tongue dorsum, erythematous gingivaPatchy brown macular pigmentation |
The etiology of aphthous ulcers (recurrent aphtous stomatitis) is still not known, but many local, systemic, immunologic, genetic, allergic, nutritional, microbiological factors, and drugs (e.g. antioxidants, non-steroidal anti-inflammatory drugs, b-blockers and immunosuppressive drugs) have been proposed as causative factors (28). Minor aphthous ulcers are round or ovoid, 2 - 4 mm in diameter, usually locating on the non-keratinized mobile mucosa. Herpetiform ulcerations are multiple small discrete ulcers that can involve any oral site. Major aphthous ulcers reach a large size, about 1 cm in diameter, and they locate at any area of the oral mucosa. They heal slowly, over 10 to 40 days.
Erythema multiforme causes multiple ulcers and/or skin lesions triggered by virus infection, drugs or hypersensitivity reaction to infectious agents or are idiopathic (29). Infectious ulcers are single or multiple, and their clinical presentation is variable (see section «Specific microbes in disease-associated conditions»). Mucosal ulcers may also be involved in cyclic neutropenia, which is a rare blood dyscrasia manifesting as cyclic depletions of neutrophils from the blood and marrow.
Lichen planus and lichenoid lesions
Around 0.5 - 4 % of adults suffer from oral lichen planus, which makes it the most common non-infectious chronic disease of the oral mucosa (30). Lichen patients are typically 30 - 60 years old, whereas it is very rare in children and young people. Despite of extensive studies, the pathogenesis of lichen planus is not known. It has been thought to be T cell-mediated cytotoxic immunoreaction triggered by intrinsic or extrinsic factor (31). Lichen planus has several clinical variations (32). The reticular form of lichen planus with white striae surrounded by erythroplakia is most common. Erythematous and erosive forms are also rather common and they often cause pain and soreness (Fig.5). Lichen lesions locate typically on buccal mucosa, gingiva and tongue, and different forms of lichen can be present at the same time. Around 15 % of lichen patients have cutaneous lesions, which typically present small, flat-topped papules on the flexor surfaces. Lichen planus has a small potential to develop into malignancy (30, 33).
Lichenoid mucositis is a common reaction on oral mucosae encountered in clinical practice (32). Lichenoid lesions can develop due to contact allergy, most often to a dental filling material or the use of some drugs, e.g. ACE-inhibitors or non-steroidal anti-inflammatory drugs. They can also develop as a result of graft-versus-host-disease or hepatitis C virus infection. Lichenoid lesions cannot be distinguished from lichen planus. Clinically, lichenoid lesions are typically unilateral and asymmetric and they locate in straight contact to a dental filling or restoration. Lichenoid lesions have been suggested to have malignant potential (30).
Oral mucosal lesions of systemic diseases
Oral mucosal manifestations may accompany systemic diseases or conditions. These include hematological diseases, autoimmune conditions, skin diseases, diseases of the gastrointestinal tract, endocrine disorders, and metabolic disorders (34). Oral mucosal findings related to systemic diseases are presented in Table 2.
Nutritional deficiencies can affect the mucous membranes. Iron deficiency is one of the most common causes of anemia. Oral mucosal changes related to iron deficiency anemia include angular cheilitis, atrophic glossitis and generalized atrophy of oral mucosa. Low levels of folate, zinc, and vitamins B1, B2, B6 and B12 have been associated with recurrent aphtous ulcers (28). Patients with eating disorders like anorexia and bulimia are particularly prone to nutritional deficiencies (35).
Oral mucosal lesions of skin diseases may clinically resemble lichen or lichenoid lesions. Systemic lupus erythematosus is a chronic autoimmune multisystem disease that shows a clear female predominance. Oral mucosal lesions include ulceration (Fig.6), mucosal erythema, and hyperkeratosis (36). Patients with discoid lupus erythematosus, a chronic skin disease, display well-demarcated skin lesions typically on sun-exposed areas. Mucosal changes are characterized by ulcerated, erythematous lesions surrounded by fine white radiating striae. Mucous membrane pemphigoid is a rare chronic vesiculobullous disease that affects predominantly oral and ocular mucous membranes. Attached gingiva can be the exclusive site of the disease. Pemphigus vulgaris is a rare mucocutaneous disease characterized by persistent and progressive skin and mucosal ulcers and vesicles.
The most common mucosal findings related to celiac disease are mucosal ulcers (28). Around 0.5 - 32 % of patients with Crohn´s disease get oral manifestations during the disease process (37). Oral symptoms of Crohn´s disease are similar to those with orofacial granulomatosis, including lip swelling, cobblestone lesions (Fig.7), mucosal ulcers with indurated borders, and gingival swelling and erythema. Orofacial granulomatous lesion is often caused by local factors, such as foreign material or inflammation. In addition to Crohn´s disease, few other systemic diseases like sarcoidosis, tuberculosis, and chronic granulomatous disease can cause granulomatous inflammation in the oral region. Granulomatosis with polyangiitis (formerly Wegener´s granulomatosis) is a rare, serious, systemic inflammatory condition of unknown etiology, which may show first signs in the oral cavity. Typical findings are red, hyperplastic, granular lesions of the attached gingiva.
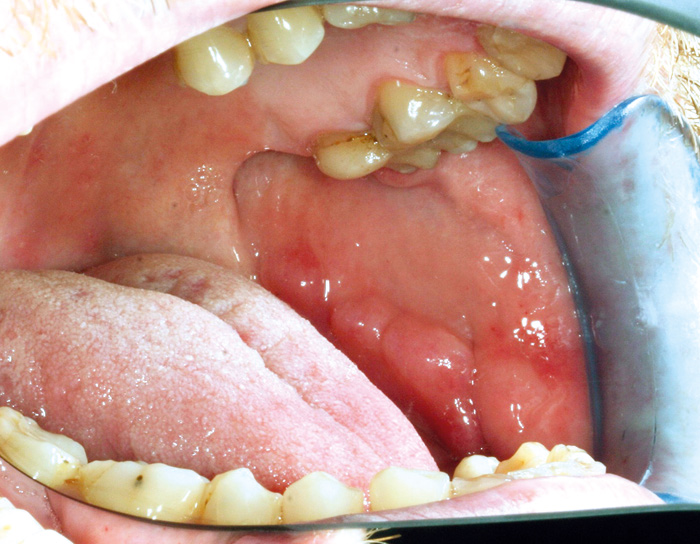
Fig. 7. Cobblestone lesion on the buccal mucosa of a 42-year-old man with Crohn´s disease. Histologically, granulomatous inflammation was detected.
Oral precancer and cancer lesions
Oropharyngeal cancer is the sixth most common cancer in the world (38). Most mucosal malignancies in the oral cavity are due to squamous cell carcinoma. Malignant salivary gland tumors, lymphomas, sarcomas, melanomas, and other malignant tumors form only a minority of oral mucosal malignancies. Survival rates of oral cancer patients have improved only modestly despite of increased knowledge of precancerous lesions and development of diagnostic methods, and remain at approximately 55 - 60 % (38).
Oral squamous cell carcinoma (OSCC) is frequently preceded by oral potentially malignant disorders (39, 40). These are leukoplakia (white lesion), proliferative verrucous leukoplakia, erythroplakia (red lesion, Fig.8), lichen planus, and lichenoid lesion. The estimated prevalence of leukoplakia is 2 - 3 % globally (41). Erytroplakia is relatively uncommon and often appears as mixed red-and-white lesions. Proliferative verrucous leukoplakia is an uncommon form of progressive multifocal leukoplakia. Snuff-induced mucosal lesions also have malignant potential (42). Typically, the lesion is asymptomatic, white, wrinkled and present on vestibular mucosae. The nature of snuff-induced mucosal lesions depends on the composition of the snuff used and the duration and frequency of snuff use.
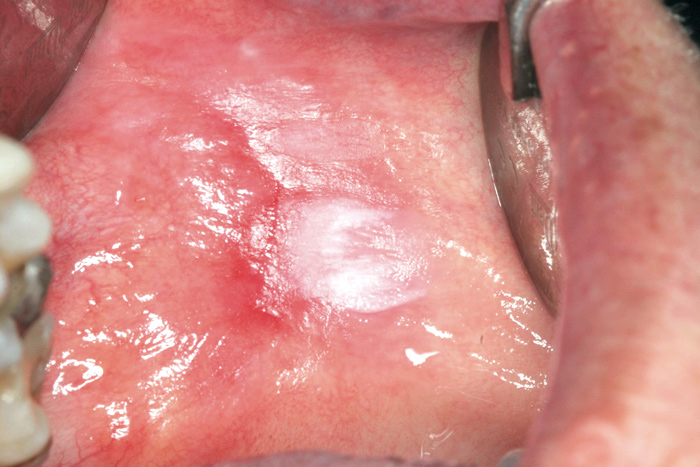
Fig. 8. Erythroleukoplakia on the lip mucosa. Histologically, dysplasia was present.
Signs and symptoms of advanced cancer lesions are generally ominous. Such cancers are often large, exophytic or deeply ulcerated and they bleed easily. For a clinician, more challenging are early cancers that present a harmless clinical picture and do not elicit any detectable symptoms. It is noteworthy that patients can initially complain discomfort, pain during mastication, and problems with swallowing and swelling in the neck area caused by lymph node metastasis.
Spesific microbes in disease-associated conditions
Bacteria
Bacteria associated with major oral polymicrobial infections, caries and periodontitis, belong to the host's resident microbiota; however, only a minor part is considered to cause harm to their host. The role of oral bacteria in the etiology of oral mucosal lesions is poorly known. It has been shown that good oral hygiene improves the symptoms of lichen planus lesions located at gingival sites, suggesting a role of dental plaque in this condition (43). Also in the case of oral lichenoid reactions, dental plaque merely than dental material may be the initiating factor (44). A report of bacterial involvement in asymptomatic oral lichen planus revealed differences in certain bacterial counts between affected and non-affected sites of the same patient as well as between patients and their controls (45). Higher counts of staphylococci and Streptococcus agalactiae, in particular, were recovered from lichen lesions. It has been suggested that the microbiota of recurrent aphtous ulcers differs markedly from that in healthy individuals; especially, the genus Prevotella was found to be dominant in aphtous lesions (46). In oral ulcers, bite marks, and on the tongue dorsum, high levels of anaerobes, e.g., Prevotella species and F. nucleatum, are frequently found (47).
Due to an «ecological catastrophe» in the host environment, the microbiota can shift out of the balance (11). Use of antibiotics and impairment of the host immune response, due to systemic diseases and medications, are among factors leading to dysbiosis. Necrotizing ulcerative gingivitis, affecting mainly young adults with an impaired immunologic status, is an acute infection with rapidly developing, painful ulcerations on interdental papillae with concomitant massive hemorrhage. Typically, elevated levels of pigmented Prevotella species, fusiforms, Selenomonas species, and spirochetes have been connected to this destructive condition on gingival mucosae (48). In immunocompromised patients and elderly, colonizing non-oral bacteria, such as aerobic gram-negative bacilli or staphylococci, cause infections on the oral mucosa (49). Potential factors exposing to mucosal infections are presented in Table 3.
Microbial groups involved |
||||
Systemic factors |
Disease 1) |
Bacteria 2) |
Fungi |
Viruses 3) |
Aging and confounding factors |
Angular cheilitis |
Aerobic GNB |
Candida |
|
Zoster |
VZV |
|||
Hormonal changes(e.g. puberty, pregnancy, menopause) |
Pubertal gingivitis |
Capnocytophaga, Prevotella |
||
Pregnancy gingivitis, pyogenic granuloma |
Prevotella |
|||
Burning mouth syndrome |
Aerobic GNB |
Candida |
||
Recurrent ulcers |
HSV-1 and -2 |
|||
Diabetes |
Candida |
|||
Malnutrition |
NUG |
Fusobacterium, Prevotella,Selenomonas,spirochetes |
||
Antimicrobial medication |
Mucositis |
Aerobic GNB |
Candida |
|
Immunosuppression (e.g. immature immunity in infancy, impaired systemic defense mechanisms, immunosuppressive medication) |
Mucositis |
Aerobic GNB, enterococci,staphylococci |
Candida |
HSV-1 and -2 |
NUG |
Fusobacterium,Prevotella, Selenomonas,spirochetes |
|||
Opportunistic infections |
Candida,other fungi |
HSV-1 and -2, VZV, EBV, CMV, KSHV, HPV |
||
Local factors |
||||
Poor oral hygiene |
Ulcerations, gingivitis,NUG |
Fusobacterium, Prevotella, Selenomonas,spirochetes |
||
Smoking |
Oral cancer |
Candida |
HPV |
|
Alcohol use |
Oral cancer |
Streptococcus anginosus |
Candida |
|
Oral cancer or neoplasia |
Candida |
|||
Corticosteroid use |
Mucositis |
Candida |
||
Xerostomia / hyposalivation |
Aerobic GNB,enterococci, staphylococci |
Candida |
||
Denture-wearing |
Denture stomatitis |
Staphylococci |
Candida |
|
1) NUG = necroticing ulcerative gingivitis
2) GNB = gram-negative bacilli
3) HSV = herpes simplex virus, VZV = varicella-zoster virus, EBV = Epstein-Barr virus, CMV = cytomegalovirus, KSHV = Kaposi´s sarcoma-associated herpesvirus, HPV = human papillomavirus
Staphylococci are typical residents of the human skin microbiota. In the oral cavity, Staphylococcus aureus has drawn attention as an opportunistic pathogen (49, 50). In these cases, S. aureus appears at high numbers, causing symptoms to the patient. Clinical situations with the common involvement of S. aureus in oral mucosal lesions include angular cheilitis, in particular, and erythematous lesions with discomfort and burning sensations (47, 49, 51). Subjects with removable dentures may suffer from denture stomatitis with S. aureus, sometimes together with Candida. According to a 3-year retrospective study (51), a fifth of the clinical oral specimens (n=5,005) sent to a diagnostic oral microbiology laboratory proved to be positive for S. aureus. Noteworthy is that methicillin-resistant S. aureus was isolated from 6 % of the 615 patients. These multiresistant isolates were often isolated from tongue specimens and were especially connected to erythema, swelling, pain or burning sensation (50). It has been suggested that methicillin-resistant S. aureus may preferentially colonize in biofilms formed on acrylic denture surfaces (51, 52). This colonization can lead to relapses after attempts to eradicate the microorganism from the oral cavity, if the eradication procedures do not include effective methods to disinfect the denture(s) (52, 53). A few staphylococcal mucositis cases, affecting the majority of oral mucosal surfaces, have been described in patients with orofacial granulomatosis and in patients with oral manifestations of Crohn's disease (54).
Aerobic gram-negative bacilli, including the so-called coliforms (lactose-positive enteric rods, such as Escherichia coli and Klebsiella species) and Pseudomonas species, are often isolated from opportunistic oral infections. In immunocompromised patients, they colonize mucosal lesions of the oral cavity, often together with Candida (47, 49). Among enterococci, Enterococcus faecalis has been linked to opportunistic infections of the oral mucosae, especially in subjects with xerostomia or hyposalivation (49). In patients with complains of discomfort on oral mucosal surfaces, aerobic enteric rods, Pseudomonas species, and enterococci are common findings, often in combinations (47). These non-oral bacteria are also frequent recoveries from oral mucosae after administration of cytotoxic drugs in adult cancer patients (55). Since chemotherapy-related mucositis causes damage to the oral mucosa, it is possible that microbes residing on mucosal surfaces contribute to this condition in a way or another. To date, there are only limited data on potential changes occurring in the oral microbiota. Recently, a prospective longitudinal cohort study, using 454 pyrosequencing of the 16S rRNA gene, followed the dynamics of bacterial communities prior to and during chemotherapy and when mucositis appeared in pediatric patients with newly diagnosed malignancies (56). At the time of diagnosis, patients who developed mucositis during chemotherapy had a higher microbial diversity and had higher levels of bacteria, representing the genus Capnocytophaga and the phyla Fusobacteria and Spirochetes, than those who did not develop mucositis. All patients had changes in the composition of the microbiota on oral mucosae during the chemotherapy. Within oral mucosal lesions, the genera Lactobacillus, Mycoplasma, and Peptostreptococcus were found in an increased abundance (56). Oral mucositis offers an entry for microorganisms to be translocated to other body sites as well as to the circulation, being an important risk for bacteremia.
Intensive research, using conventional culture and molecular techniques, has enlightened the involvement of oral species in OSCC. Compared to biofilms on healthy oral mucosae, carcinoma surfaces seem to harbor higher levels of many anaerobic species, such as Fusobacterium, Porphyromonas, Prevotella, and Veillonella (57). Also microaerophilic Streptococcus anginosus has been suggested as an important finding in OSCC tissues but hardly in other oral cancer types (58). In these cases, dental plaque was considered of being the main source of S. anginosus. In a recent study of Pushalkar et al. (59), tissue samples were taken from tumor and healthy sites of 10 subjects with OSCC, targeting to differences in bacterial profiles and their potential involvement in tumor pathogenesis. Using an advanced molecular method, a shift in bacterial colonization was demonstrated; many streptococcal species (S. salivarius, S. gordonii, S. parasanguinis), Gemella species (G. haemolysans, G. morbillorum, G. sanguinis), Johnsonella ignava, and Peptostreptococcus stomatis, and some uncultivated oral taxa proved to be highly associated with tumor sites but Granulicatella adiacens with non-tumor sites (59). Although these species are residents of the mouth, they can behave as pathogens when the homeostasis within the microbiota is disturbed and moves to a dysbiotic state. Bacterial shifts are reflected by their significantly increased salivary levels in OSCC patients and, if so, could be used as diagnostic indicators measured from saliva (60).
Some non-oral infections caused by bacteria, such as tuberculosis, gonorrhea, and syphilis can have manifestations on oral mucosae, which need to keep in mind in differential diagnostics (49). The presence of their infectious agents, Mycobacterium tuberculosis, Neisseria gonorrhoeae, and Treponema pallidum, respectively, in oral lesions and saliva can be contagious. There has been a reappraisal of these infectious diseases in Western countries. For instance, the incidence of syphilis has considerably increased during the past 10 years in Germany and Switzerland, and the initial suspicion of the reported cases came from the dentist on the basis of its oral manifestations (61).
Fungi
Oral fungal infections are predominantly caused by Candida species (62). C. albicans is most common, but several other species, including C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis are also frequently isolated (63). In addition to growing on mucosal surfaces, Candida is effectively forming biofilms on teeth and artificial materials, such as dentures. Defects of local or systemic immune defense expose to Candida infection (Table 3). Predisposing factors for fungal infection include the use of antibiotics or inhaled corticosteroids, which may disturb the homeostasis of the bacterial microbiota of the mouth. Also systemic immunosuppressive diseases, such as advanced HIV infection, can enable opportunistic infections on oral mucosae. In severely immunocompromised patients, also certain saprophytic fungi, including Aspergillus and Mucor species, may cause infections of the oral mucosa and invade the neighboring tissues (62).
Oral burning, pain, and taste alterations are typical symptoms of Candida infection. Clinically, an acute candidiasis can be suspected, if erythematous mucosa or mucosal pseudomembrane (trush) covering erythematous mucosa is observed (62). On tongue, the infection can cause papilla atrophy. Chronic forms of Candida infection may appear as leukoplakic or hyperplastic candidiasis typically manifesting as leukoplakia- or fibroma-like mucosal thickening. A rare manifestation of oral yeast infection is chronic mucocutaneous candidiasis. Chronic Candida infections have been associated with malignant transformation, which may be at least partly attributed to production of carcinogenic acetaldehyde by yeasts (62, 63). Furthermore, in infections, such as angular cheilitis, median rhomboid glossitis, and denture stomatitis, Candida often plays a role together with bacteria, especially staphylococci (47, 49, 50). Linear gingival erythema, predominantly diagnosed in HIV-infected individuals, is also associated with Candida (62).
Viruses
Viral infections can manifest in the oral cavity either as blisters or ulcers, hyperkeratosis or vascular lesions.
Herpes simplex virus type 1 (HSV-1) is typically contracted in close contact to infectious secretions or lesions. In the oral region, primary HSV-1 infection (infection following the first contact with HSV-1) is usually subclinical or so mild that it remains unrecognized. In only 1 - 10 % of individuals, infection manifests as painful gingivostomatitis with small ulcers and blisters distributed throughout the oropharynx, accompanied by fever and cervical lymphadenopathy (64). In recent years, HSV-1 transmission has become less frequent during childhood (65). Instead, the first contact with HSV-1 occurs later in life and, therefore, primary infections are increasingly diagnosed in teenagers and adults.
During primary infection, virus is transported to the sensory ganglion corresponding to the site of infection, and a latent infection is established. Following HSV reactivation from latency, asymptomatic shedding into saliva or recurrent infection ensues. Recurrent infection is more limited and manifests as labial herpes or localized infection of the mucosa or the skin. Immunosuppression, stress, exposure to sunlight, tissue trauma, and hormonal changes are known triggers for HSV-1 reactivation. Also HSV type 2 (HSV-2) can infrequently be identified in the oral cavity (66). HSV-1 and HSV-2 are both well-established triggers for erythema multiforme (67).
Another herpesvirus, varicella-zoster virus (VZV), can cause blisters or ulcers on the oral mucosa during primary VZV infection or varicella. Zoster is the manifestation of VZV infection following the reactivation of the virus from the sensory ganglion (68). The likelihood for zoster is increasing with aging. It can be preceded by intense pain resembling toothache in the area of developing zoster infection. Zoster rash is classically limited to the body midline in the area of 1 - 3 nerve branches at a time. In some patients, post-herpetic neuralgia develops and may persist for weeks or months requiring treatment with neuropathic pain medication.
Oral reactivation of both HSV and VZV typically manifests on the attached mucosa, which helps to distinguish these infections, for example, from aphtae. Differentiating these two viruses reliably from each other requires diagnostic tests. Differential diagnosis of HSV and VZV stomatitis includes enterovirus infections affecting the oral region, namely hand-foot and mouth disease and herpangina (66). In herpangina, blisters and ulcers are limited to the soft palate and the tonsils, whereas in hand-foot and mouth disease these can be distributed throughout the oropharyngeal mucosa, and papules and blisters erupt also on the skin, particularly on the hands and feet. Other symptoms are fever, malaise, and diarrhea. Non-infectious differential diagnosis of oral blisters and ulcers include aphtae, erythema multiforme, neutropenic ulcers, bullous lichen planus, bullous pemphigoid, and pemphigus.
More than 150 genotypes of human papillomaviruses (HPV) have been identified. They are divided in high- and low-risk types depending on their risk for causing carcinoma. HPV genotypes also differentially infect keratinocytes of either mucosa, skin or both mucosa and skin. In the oral cavity, HPV has been detected in approximately 1 - 20 % of asymptomatic patients depending on the detection method (69, 70). HPV infection can be asymptomatic or manifest as benign warts or condylomas. HPV infection should be suspected, if papillomatous epithelial overgrowth is observed. HPV genotypes 13 and 32 cause specific smooth-surfaced mucosa-colored papulonodular or plebbed-surfaced whitish papillomatous lesions, called focal epithelial hyperplasia in genetically predisposed subjects (71).
HPV causes approximately 20 % of oral carcinomas and 60 - 80 % of oropharyngeal carcinomas (72). The risk of HPV-associated oral cancer is most frequently related to the genotypes 16 and 18, but also other genotypes, including low-risk genotypes HPV 6 and 11, have been detected in oral cancer (70). HPV-associated head and neck cancer patients typically do not possess the classical risk factors for oral cancer (longtime smoking and alcohol abuse) and they are younger (74). Their prognosis is better compared to HPV-negative cancer patients. HPV has also been detected in potentially malignant disorders, such as leukoplakia and oral lichen planus (33).
All above-discussed viral infections are common in immunocompetent individuals, but become increasingly frequent and are atypically severe in immunocompromised patients (Table 3), such as patients with advanced HIV infection (75), transplant patients or patients with hematologic malignancies (66). Also asymptomatic shedding of herpesviruses into saliva is increased in these patients
Certain oral manifestations of viral infections are practically only observed in immunocompromised individuals (66). These include oral hairy leukoplakia, Kaposi´s sarcoma, and cytomegalovirus (CMV)-induced oral ulcers. Hairy leukoplakia is a manifestation of Epstein-Barr virus (EBV) reactivation when bilateral, white, vertically corrugated leukoplakia in the posterior part of the tongue is a characteristic finding. This condition is usually asymptomatic and does not need to be treated. However, hairy leukoplakia can be secondarily infected with Candida. EBV is an oncogenic virus and is known to associate with certain subtypes of lymphoma and nasopharyngeal carcinoma (66). Kaposi´s sarcoma is a lymphoid vascular neoplasia caused by human herpesvirus 8 or Kaposi´s sarcoma-associated herpesvirus. In the oral cavity, it manifests as purple tumor- or vascular-like lesions. Diagnosis of oral hairy leukoplakia, Kaposi´s sarcoma or CMV-associated ulcer in a previously healthy patient always necessitates investigations regarding the underlying cause of immunosuppression.
Diagnostics of oral mucosal lesions
Clinical examination
A systematic and thorough investigation of oral mucosae is essential in the beginning of every dental treatment period (76). Tongue has to be drawn out to be able to see its posterior lateral borders and lingual tonsils. Clinical photographs of oral mucosal lesions facilitate the consultation of lesions with a specialist. In addition, extraoral areas must be examined. Deviations in the face and head area, enlarged lymph nodes and salivary glands, and lip and facial skin lesions must be noticed. Poorly fitting dentures or problems with eating and swallowing may be the first signs of oral malignancy.
Non-invasive diagnostic methods based on polarimetry techniques have been developed (77). These methods provide useful tools, for instance, for the detection of small mucosal lesions and to define surgical margins. Adequate investigation of a patient with oral mucosal lesions can also require blood and skin testing. Referral to a dermatologist or specialist in internal medicine may be indicated.
Biopsies for histology
Many mucosal lesions can be diagnosed by a general dentist. When complicated or serious diagnosis is suspected, the diagnosis is doubtful or if the patient has severe medical problems, a referral to a specialist is indicated. Table 4 presents indications for oral biopsy. Oral mucosal lesions that do not disappear within 2 - 3 weeks must be biopsied. In case of wide lesions, more than one biopsy is needed to have an adequate picture of the mucosal disease condition. In general, sites with induration, redness or ulcerations are usual indications for biopsy. In addition, large (2 cm in diameter) and multifocal lesions should be carefully investigated to rule out malignancy. Of ulcers, periulcerative mucosa is necessary to be included in the biopsy specimen. Lesions smaller than 1 cm in diameter should be excised (removed completely) for biopsy. The decision to use a punch device or scalpel is based on the anatomical site and the clinician´s preference. Routine histologic samples are fixed in formalin. A fine needle biopsy for oral mucosal lesions is not common but may be useful, for instance, in the diagnostics of salivary gland tumors.
· White lesions (leukoplakia) |
· Erythematous lesions (erythroplakia) |
· Ulcers of lip, tongue and other mucosal areas, that have not healed in 2 - 3 weeks - also tooth extraction sockets that do not heal |
· Mucosal hyperplasia lesions |
· Nodular lesions |
· Pigmented lesions - melanoma has to be ruled out, even though it is very rare in oral mucosa |
· Vascular lesions - if there is a risk of uncontrolled bleeding the patient should be referred to hospital for biopsy |
· Labial minor salivary gland biopsy to confirm Sjögren´s syndrome |
· Periapical lesions in connection with tooth extractions |
· All radiographically radiolucent areas of jaws |
· Lesions with significantly changed clinical appearance or symptoms, although previously already diagnosed/biopsied |
Biopsies for direct immunofluorescence examination |
· Qualitative technique to detect immune deposits (antibodies and/or complement) in the tissues |
· In the diagnosis of oral lesions of skin diseases, particularly of vesiculobullous disorders such as pemphigoid and pemphigus |
· Lesional or perilesional tissue |
· Submitted immediately to the laboratory for freezing /submitted in solution compatible with immunofluorescence technique (Michel´s solution) |
The clinical diagnosis of lichen planus should be confirmed by tissue biopsy, especially, if reddish or erosive areas are present (32). The histologic picture of lichen shows a dense infiltrate of lymphocytes at the epithelium-connective tissue interface and epithelial basal layer degeneration. Certain histological features, such as deep inflammatory cell infiltrate, perivascular infiltrates, and the presence of plasma cells and eosinophils, are mainly associated with lichenoid lesions. The clinical and histologic picture of erosive or erythematous lichen may resemble bullous pemphigoid, pemphigus, epidermolysis bullous acquisita, dermatitis herpetiformis, erythema multiforme, and acute lesions of lupus (32). Immunofluorescence techniques are especially useful in the differential diagnostics of oral mucosal lesions of skin diseases (see Table 2). Mucous membrane pemphigoid shows autoantibodies against basement membrane proteins. Histologically, subepithelial bullae and chronic inflammation can be detected. Pemphigus vulgaris is characterized by autoimmune reaction to intercellular keratinocyte protein forming intraepithelial bullae. Subepithelial edema and deep infiltration of lymphocytes in a perivascular orientation are typical features of lupus. Patients with mucosal lesions of skin diseases and other systemic diseases have to be followed regularly and new biopsies need to be taken, especially, if there are changes in the clinical picture of the disease or if dysplasia is present.
Patients with multiple recurrent oral ulcers, gingival swellings, and erythema and/or mucosal cobblestone lesions should be carefully examined to find out possible systemic disease behind oral lesions. Orofacial granulomatosis is confirmed by a tissue biopsy (37). Histologically, granuloma formation with lymphocytes and epithelioid histiocytes with or without multinucleated giant cells are seen. Hematologic and gastrointestinal as well as other investigations may be required to exclude systemic diseases. A typical histopathologic feature of granulomatosis with polyangiitis is granulomatous inflammation with necrotizing vasculitis.
After clinical diagnosis of leukoplakia or erythroplakia, all predisposing factors should be eliminated. Smoking-induced leukoplakia may heal after quitting smoking. Snuff-induced mucosal lesions may also heal after quitting snuff use. If leukoplakia or erythroplakia has not disappeared after 2 - 3 weeks' follow-up, the lesion should be studied by tissue biopsy. The histologic picture of leukoplakia can vary from benign epithelial hyperkeratosis, dysplasia and carcinoma in situ to OSCC. Increasing degrees of dysplasia are designated as mild, moderate or severe. For clinicians, it is challenging to predict which leukoplakia lesions will progress into cancer. Main risk factors for leukoplakia transformation are the male gender, long duration of the lesion, non-homogenous appearance of the lesion, tongue/floor of the mouth/soft palate location of the lesion, size of 200mm2 and dysplasia present (78). Erythroplakia and erythroleukoplakia show epithelial atrophy and are also more likely to represent dysplasia or malignancy (39, 40). It is generally accepted that the more severe the epithelial changes are, the more likely a lesion will progress to cancer. Proliferative verrucous leukoplakia has a high risk of malignant transformation and these patients need a careful follow-up.
Most OSCCs are moderately or well-differentiated lesions. Invasion of tumor cell nests into subadjacent structures, keratins pearls, and individual cell keratinization are typical histologic features. Verrucous carcinoma shows a hyperplastic lesion with broad, pushing rete ridges and well-differentiated epithelial cells. Sometimes, the diagnostics of malignant lesions can be very challenging. For example, the pseudoepithelial hyperplasia detected within chronic Candida infection may closely resemble carcinoma lesion. On the other hand, the pathology report can deny malignancy, even though the clinical diagnosis is cancer. Then, the histologic samples should be re-checked and/or a re-biopsy is indicated.
Microbiologic samples
In situations where the patient has symptoms and there is a mucosal lesion, microbiologic testing is often indicated (Table 5). Saliva and oral rinses are commonly used specimen types for identifying causative agents of certain oral infections and systemic diseases (49, 79). However, these specimens are not optimal for diagnosing site-specific mucosal lesions. In such cases, it is preferable to take scraping, filter paper imprint or mucosal swab samplings (49, 80; Table 5).
Sampling techniques |
Bacteria |
Fungi |
Viruses |
Local/ site-specific samplings on mucosa |
|||
· scraping |
P |
P |
|
· filter paper imprint |
P |
P |
|
· swab |
P |
P |
P |
· biopsy |
P |
P |
|
General samplings |
|||
· saliva |
P |
P |
|
· oral rinse |
P |
P |
|
Laboratory techniques |
|||
Microscopy |
|||
· light, native or with certain reagents |
P |
P |
|
· darkfield (e.g., spirochetes) |
P |
||
· immunofluorescence with specific antibodies |
P |
||
· histology with special stains or immunohistology |
P |
P |
P |
Culture |
|||
· non-selective media |
P |
||
· selective media |
P |
P |
|
· viral culture |
P |
||
Molecular biology |
|||
· PCR |
P |
P |
P |
· DNA-DNA hybridization («checkerboard») |
P |
||
Serology (antibody detection; e.g. syphilis) |
P |
Although Candida infections may be diagnosed even clinically, it is preferable that a culture specimen is taken to confirm the diagnosis and when needed, to differentiate between fungal and bacterial infection. A culture sample for the identification of Candida species and, possibly, for the sensitivity testing is strongly recommended in the cases of treatment failure, severely immunosuppressed patients, and if the patient needs antifungal treatment frequently. Swab or imprint samples are taken from the mucosal lesion suspected to represent Candida infection, whereas saliva and oral rinse samples can be used as a sampling method for diagnosis of more generalized oral infection (63). By culture, an approximate amount of Candida in the sample can be counted, and the identification of isolates to the species level is performed by further testing, e.g. using chromogenic media for culture. Consideration is needed when interpreting the culture results in order to differentiate colonization from infection. For hyperplastic candidiasis and infections caused by other fungi than Candida, a smear and biopsy specimens are recommended for culture and microscopic and histologic examination. The detection of Candida hyphae penetrating the upper layers of the epithelium and presence of inflammation in any histologic sample signifies Candida infection. Also other oral diseases, such as lichen planus or even oral carcinoma, can cause candidiasis-like symptoms. Furthermore, it is possible that these lesions are colonized or infected with Candida. It should be noted that in such cases the underlying mucosal disease may be missed, if only microbiologic sampling is used for diagnosis.
Specimens for viral culture, antigen detection, and PCR are taken as a swab sample from an ulcer or a blister. Infected cells need to be incorporated in the swab to ensure sufficient cellular material for analysis. Poor sampling technique severely decreases the sensitivity of especially viral culture and antigen detection assays. Viral cell culture is useful for detecting HSV, VZV, and enteroviruses. Some laboratories use antigen detection by immunofluorescence microscopy for diagnosis of HSV and VZV. PCR can be used for the detection of all viruses from swab or biopsy samples; however, particularly with swab samples, care must be taken not to mistakenly interpret viral shedding as infection. Viral infections may be diagnosed histologically from a biopsy sample. This approach is especially indicated for diagnosis of HPV-associated warts, CMV-induced ulcers, EBV-associated oral hairy leukoplakia, and Kaposi´s sarcoma. It is also possible to perform genotyping of HPV from tissue and swab samples. Because dysplastic changes may occur in persisting wart-like HPV-infections, these lesions should be surgically removed for histologic diagnosis.
Concluding remarks
All health care professionals should examine the oral mucosa regularly. Oral mucosal lesions can rarely be diagnosed on their clinical appearance alone. Biopsies from all potentially malignant lesions should be taken to rule out dysplasia or oral cancer. Early diagnosis of oral cancer is essential for improving the prognosis. Microbiologic sampling is often required for diagnosing infections on oral mucosae. In cases of systemic diseases manifesting on the oral mucosa, consultation of or referral to medical specialists is recommended.
References
Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol. 2000 2006; 42: 80 - 7.
Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013; 69: 137 - 43.
Könönen E. Development of oral bacterial flora in young children. Ann Med. 2000; 32: 107 - 12.
Haraldsson G, Holbrook WP, Könönen E. Clonal persistence of oral Fusobacterium nucleatum in infancy. J Dent Res. 2004; 83: 500 - 4.
Hohwy J, Reinholdt J, Kilian M. Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun. 2001; 69: 6055 - 63.
Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011; 4: 22.
Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy «core microbiome» of oral microbial communities. BMC Microbiol. 2009; 9: 259.
Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010; 4: 962 - 74.
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005; 43: 5721 - 32.
Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003; 30: 644 - 54.
Marsh PD, Percival RS. The oral microflora--friend or foe? Can we decide? Int Dent J 2006; 56(Suppl 1): 233 - 9.
Preza D, Olsen I, Willumsen T, Grinde B, Paster BJ. Diversity and site-specificity of the oral microflora in the elderly. Eur J Clin Microbiol Infect Dis. 2009; 28: 1033 - 40.
Sachdeo A, Haffajee AD, Socransky SS. Biofilms in the edentulous oral cavity. J Prosthodont 2008; 17: 348 - 56.
Krom BP, Kidwai S, Ten Cate JM. Candida and other fungal species: forgotten players of healthy oral microbiota. J Dent Res. 2014; 93: 445 - 51.
Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010; 6: e1000713.
Mumcu G, Cimilli H, Sur H, Hayran O, Atalay T. Prevalence and distribution of oral lesions: a cross-sectional study in Turkey. Oral Dis. 2005; 11: 81-7.
Axéll T. A prevalence study of oral mucosal lesions in an adult Swedish population. Odontol Revy Suppl. 1976; 36: 1 - 103.
Cebeci AR, Gül?ahi A, Kamburoglu K, Orhan BK, Ozta? B. Prevalence and distribution of oral mucosal lesions in an adult Turkish population. Med Oral Patol Oral Cir Bucal. 2009; 14: E272 - 7.
Shulman JD, Beach MM, Rivera-Hidalgo F. The prevalence of oral mucosal lesions in U.S. adults: data from the Third National Health and Nutrition Examination Survey, 1988 - 1994. J Am Dent Assoc. 2004; 135: 1279 - 86.
Ali M, Joseph B, Sundaram D. Prevalence of oral mucosal lesions in patients of the Kuwait University Dental Center. Saudi Dent J. 2013; 25: 111 - 8.
Splieth CH, Sümnig W, Bessel F, John U, Kocher T. Prevalence of oral mucosal lesions in a representative population. Quintessence Int. 2007; 38: 23 - 9.
Kovac-Kovacic M, Skaleric U. The prevalence of oral mucosal lesions in a population in Ljubljana, Slovenia. J Oral Pathol Med. 2000; 29: 331 - 5.
Jahanbani J, Sandvik L, Lyberg T, Ahlfors E. Evaluation of oral mucosal lesions in 598 referred Iranian patients. Open Dent J. 2009 27; 3: 42 - 7.
Reichart PA. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dent Oral Epidemiol. 2000; 28: 390 - 8.
Pentenero M, Broccoletti R, Carbone M, Conrotto D, Gandolfo S. The prevalence of oral mucosal lesions in adults from the Turin area. Oral Dis. 2008; 14: 356 - 66.
Martori E, Ayuso-Montero R, Martinez-Gomis J, Vi?as M, Peraire M. Risk factors for denture-related oral mucosal lesions in a geriatric population. J Prosthet Dent. 2014; 111: 273 - 9.
Ali M, Sundaram D. Biopsied oral soft tissue lesions in Kuwait: a six-year retrospective analysis. Med Princ Pract. 2012; 21: 569 - 75.
Akintoye SO, Greenberg MS. Recurrent aphtous stomatitis. Dent Clin North Am 2014; 58: 281 - 97.
Farthing P, Bagan JV, Scully C. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis. 2005; 11: 261 - 7.
Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008; 46: 15 - 21.
Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus - a review. J Oral Pathol Med. 2010; 39: 729 - 34.
Schlosser BJ. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol Ther. 2010; 23: 251 - 67.
Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011; 17(Suppl 1): 58 - 72.
Islam NM, Bhattacharyya I, Cohen DM. Common oral manifestations of systemic disease. Otolaryngol Clin North Am. 2011; 44: 161 - 82.
Schlosser BJ, Pirigyi M, Mirowski GW. Oral manifestations of hematologic and nutritional diseases. Otolaryngol Clin North Am. 2011; 44: 183 - 203.
Khatibi M, Shakoorpour AH, Jahromi ZM, Ahmadzadeh A. The prevalence of oral mucosal lesions and related factors in 188 patients with systemic lupus erythematosus. Lupus. 2012; 21: 1312 - 5.
Rowland M, Fleming P, Bourke B. Looking in the mouth for Crohn´s disease. Inflamm Bowel Dis. 2010; 16: 332 - 7.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; 45: 309 - 16.
Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007; 36: 575 - 80.
Rhodus NL, Kerr AR, Patel K. Oral cancer: Leukoplakia, premalignancy, and squamous cell carcinoma. Dent Clin North Am. 2014; 58: 315 - 40.
Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol 2003; 39: 770 - 80.
Roosaar A, Johansson AL, Sandborgh-Englund G, Axéll T, Nyrén O. Cancer and mortality among users and nonusers of snus. Int J Cancer. 2008; 123: 168 - 73.
Holmstrup P, Schiøtz AW, Westergaard J. Effect of dental plaque control on gingival lichen planus. Oral Surg Oral Med Oral Pathol. 1990; 69: 585 - 90.
Bäckman K, Jontell M. Microbial-associated oral lichenoid reactions. Oral Dis. 2007; 13: 402 - 6.
Bornstein MM, Hakimi B, Persson GR. Microbiological findings in subjects with asymptomatic oral lichen planus: a cross-sectional comparative study. J Periodontol. 2008; 79: 2347 - 55.
Marchini L, Campos MS, Silva AM, Paulino LC, Nobrega FG. Bacterial diversity in aphtous ulcers. Oral Microbiol Immunol. 2007; 22: 225 - 31.
Dahlén G, Blomquist S, Carlén A. A retrospective study on the microbiology in patients with oral complaints and oral mucosal lesions. Oral Dis. 2009b; 15: 265 - 72.
Gmür R, Wyss C, Xue Y, Thurnheer T, Guggenheim B. Gingival crevice microbiota from Chinese patients with gingivitis or necrotizing ulcerative gingivitis. Eur J Oral Sci. 2004; 112: 33 - 41.
Dahlén G. Bacterial infections of the oral mucosa. Periodontol. 2000 2009; 49: 13 - 38.
Smith AJ, Jackson MS, Bagg J. The ecology of Staphylococcus species in the oral cavity. J Med Microbiol. 2001; 50: 940 - 6.
Smith AJ, Robertson D, Tang MK, Jackson MS, MacKenzie D, Bagg J. Staphylococcus aureus in the oral cavity: a three-year retrospective analysis of clinical laboratory data. Br Dent J. 2003; 195: 701 - 3.
Lee D, Howlett J, Pratten J, Mordan N, McDonald A, Wilson M, et al. Susceptibility of MRSA biofilms to denture-cleansing agents. FEMS Microbiol Lett. 2009; 291: 241 - 6.
Rossi T, Peltonen R, Laine J, Eerola E, Vuopio-Varkila J, Kotilainen P. Eradication of the long-term carriage of methicillin-resistant Staphylococcus aureus in patients wearing dentures: a follow-up of 10 patients. J Hosp Infect. 1996; 34: 311 - 20.
Gibson J, Wray D, Bagg J. Oral staphylococcal mucositis: A new clinical entity in orofacial granulomatosis and Crohn´s disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89: 171 - 9.
Napeñas JJ, Brennan MT, Bahrani-Mougeot FK, Fox PC, Lockhardt PB. Relationship between mucositis and changes in oral microflora during cancer chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103: 48 - 59.
Ye Y, Carlsson G, Agholme MB, Wilson JAL, Roos A, Henriques-Normark B, et al. Oral bacterial community dynamics in paediatric patients with malignancies in relation to chemotherapy-related oral mucositis: a prospective study. Clin Microbiol Infect. 2013; 19: E559 - 67.
Nagy KN, Sonkondi I, Szöke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998; 34: 304 - 8.
Sasaki M, Yamaura C, Ohara-Nemoto Y, Tajika S, Kodama Y, Ohya T, et al. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005; 11: 151 - 6.
Pushalkar S, Ji X, Li Y, Estilo C, Yegnanarayana R, Singh B, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012; 12: 144.
Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Trans Med. 2005; 3: 27.
Hertel M, Matter D, Schmidt-Westhausen AM, Bornstein MM. Oral syphilis: A series of 5 cases. J Oral Maxillofac Surg. 2014; 72: 338 - 45.
Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol. 2000 2009; 49: 39 - 59.
Rautemaa R, Ramage G. Oral candidosis--clinical challenges of a biofilm disease. Crit Rev Microbiol. 2011; 37: 328 - 36.
Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001; 357: 1513 - 8.
Pebody RG, Andrews N, Brown D, Gopal R, De Melker H, François G, et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. 2004; 80: 185 - 91.
Slots J. Oral viral infections. Periodontology. 2000 2009; 49: 60 - 86.
Farthing P, Bagan JV, Scully C. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis 2005; 11: 261 - 7.
Cohen JI. Herpes zoster. N Engl J Med. 2013; 369: 1766 - 7.
Kellokoski JK, Syrjänen SM, Chang F, Yliskoski M, Syrjänen KJ. Southern blot hybridization and PCR in detection of oral human papillomavirus (HPV) infections in women with genital HPV infections. J Oral Pathol Med. 1992; 21: 459 - 64.
Kreimer AR, Bhatia RK, Messeguer AL, González P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis. 2010; 37: 386 - 91.
Said AK, Leao JC, Fedele S, Porter SR. Focal epithelial hyperplasia - an update. J Oral Pathol Med. 2013; 42: 435 - 42.
Rautava J, Syrjänen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head and Neck Pathol. 2012; 6 (Suppl 1): S3 - 15.
Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005; 14: 467 - 75.
Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008; 100: 407 - 20.
Reznik DA. Oral manifestations of HIV disease. Top HIV Med. 2005; 13: 143 - 8.
Video on Oral examination in Oral cancer, Current care guideline. Working group set up by the Finnish Medical Society Duodecim and the Finnish Dental Society Apollonia. Helsinki: Finnish Medical Society Duodecim, 2012. Available online at: www.kaypahoito.fi
López-Jornet P, De la Mano-Espinosa T. The efficacy of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice: an update. Int J Dent Hyg. 2011; 9: 97 - 100.
Scully C. Challenges in predicting which oral mucosal potentially malignant disease will progress to neoplasia. Oral Dis. 2014; 20: 1 - 5.
Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013; 26: 781 - 91.
Rusanen P, Siikala E, Uittamo J, Richardson M, Rautemaa R. A novel method for sampling the microbiota from the oral mucosa. Clin Oral Investig. 2009; 13: 243 - 6.
Adress: Jaana Willberg, Institute of Dentistry, University of Turku, Lemminkäisenkatu 2, FIN-20520 Turku, Finland. E-mail: jaana.willberg@utu.fi
Artikkelen har gjennomgått ekstern faglig vurdering.
Willberg J, Välimaa H, Gürsoy M, Könönen E. Diagnostics of oral mucosae. Histology and microbiology - clinical relevance. Nor Tannlegeforen Tid. 2015; 125: 120-33.
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Willberg J, Välimaa H, Gürsoy M, Könönen E. Diagnostics of oral mucosae. Nor Tannlegeforen Tid. 2015;125:120-33. doi:10.56373/2015-2-11