Common oral mucosal lesions
Headlines
All general dental practitioners experience common oral mucosal diseases in their practice.
Once a correct diagnosis of the oral lesion is established, it is possible to acquire information about management strategies through different information sources.
The rationale for choosing the lesions included in the present article is that they are the most common oral mucosal diseases that general practitioners experience in their practice [1][2][3]. The key problem is to reach a correct diagnosis. Therefore, the primary goal of the present paper is to detail the clinical and histopathological characteristics and other paraclinical examinations (when appropriate). Once a correct diagnosis is established, it is often possible to acquire information about appropriate management strategies through several different information sources. The presented lesions should be seen in perspective of paper IV of the present volume, where important differential diagnoses to the lesions are portrayed.
Angular cheilitis
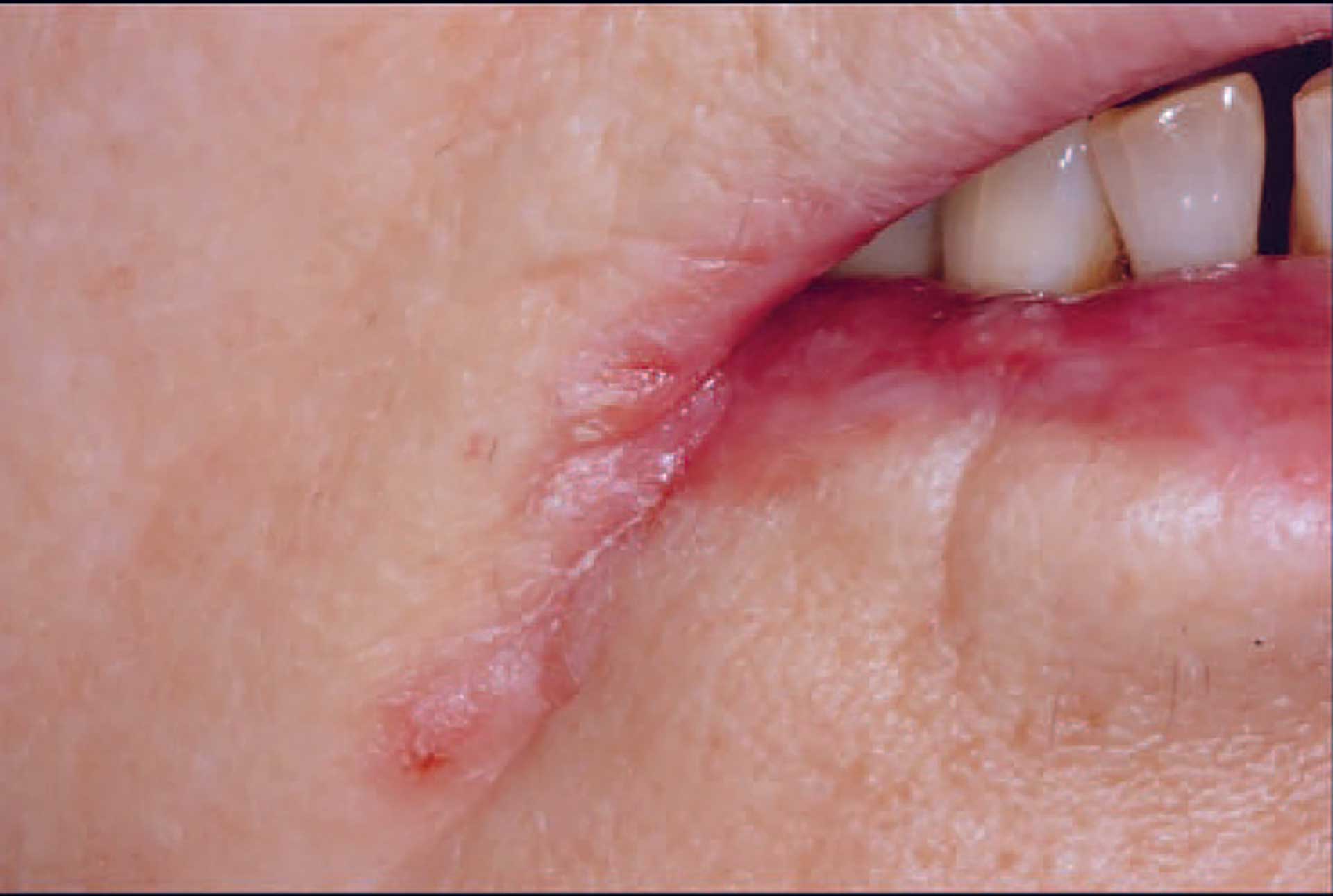
Figure 1. Angular cheilitis.
This lesion is primarily not a diagnostic problem since it has a unique location (figure 1). However, an increased understanding of the etiology and how the lesion is maintained is important for proper management [4].
Clinical features
Older people with extended angular folds probably have equal potential to develop angular cheilitis regardless of whether they have dentures, but denture wearers specifically have a microflora that facilitates the development of the infection. Thus, the theory has been advocated that it is not the decrease in the vertical dimension per se that is of greatest importance but the presence of an ageing tissue. Angular cheilitis in younger patients is characterized by a single rhagade limited to the corner of the mouth. Most of these patients report an atopic constitution and cutaneous disorders [4] [5].
Aetiology and pathogenesis
The pathogenesis begins with a fissure at the corner of the mouth due to aging of the skin or atopy. The rhagade is then infected by fungi or Staphylococcus aureus. Older individuals are usually infected by Candida albicans, while younger individuals more often contract the bacterial infection [4].
Treatment
The treatment is a combination of both antifungal and antibacterial drugs, such as miconazole. The drug may also be combined with hydrocortisone. It is important that the patient does not apply the drug with a finger since the risk of reinfection is high because Staphylococcus aureus can be found on the skin. Instead, cotton tops or other modes of application should be used. Once the infection has healed, it is important to keep the skin lubricated with a softening ointment to avoid relapses. Again, cotton tops or other modes of application are preferred, at least in the initial stages [6].
Recurrent herpes labialis
Recurrent herpes labialis (RHL) is one of the most common human viral infections worldwide [7] [8].
Clinical features
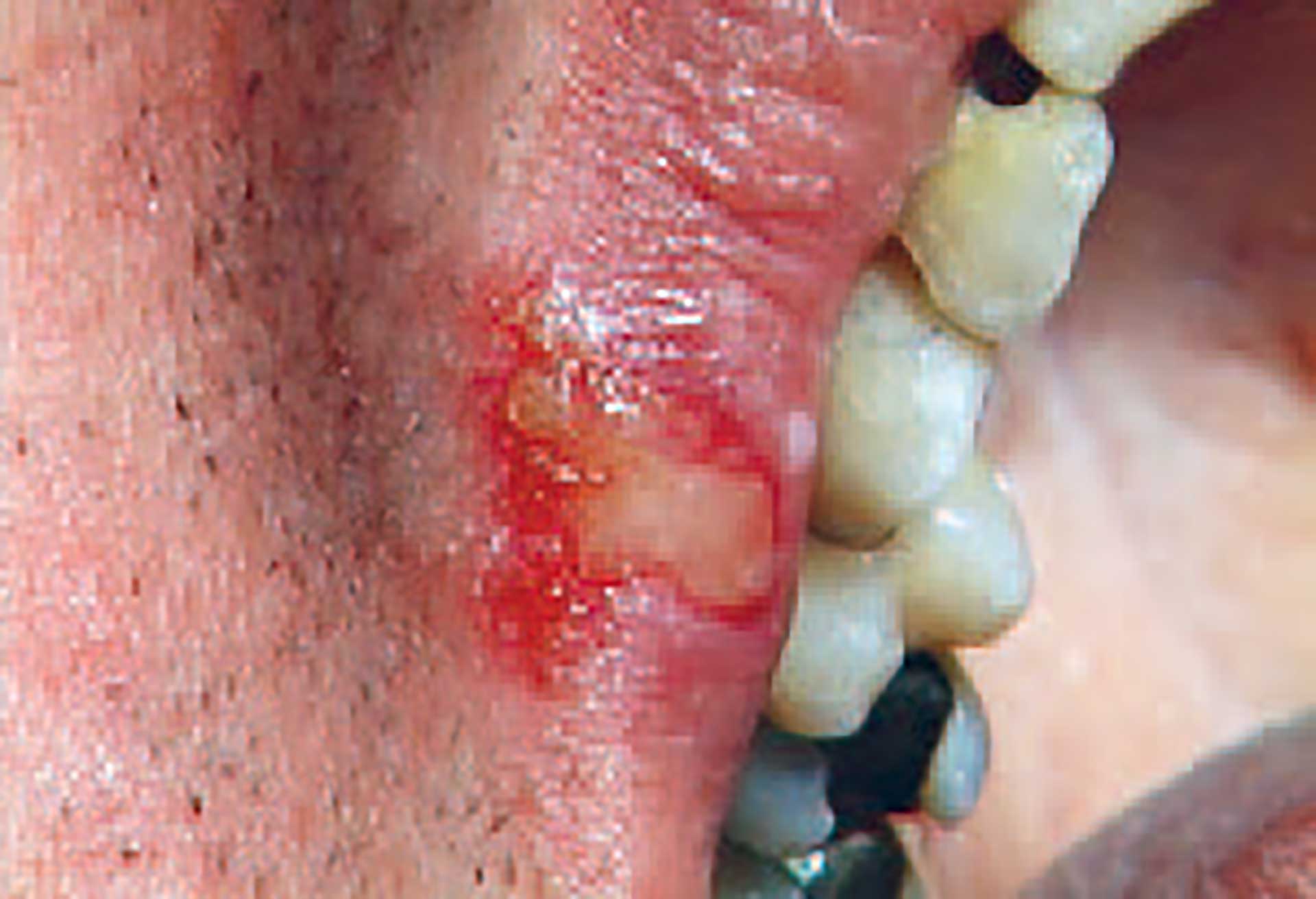
Figure 2. Recurrent herpes labialis.
The most common location of RHL is the labial vermillion border (figure 2), appearing as cold sores, while intraoral infection is uncommon in otherwise healthy persons. The symptoms begin with a sensation of burning pain and itchiness and progress to a painful lesion which manifest as clusters of small and often “thin-walled” microvesicles. These intraepithelial lesions may progress to pustules, erosions, and ulcerations and coalesce into larger irregular ulcers (diameter 0.5 - 1 cm) surrounded by an erythematous zone. Lesions eventually develop a crust and then resolve normally within 10 to 14 days and heal without scarring [9].
Aetiology and pathogenesis
RHL is caused by the reactivation of herpes simplex virus type 1 (HSV-1) in the trigeminal sensory ganglion. Primary HSV-1 infection is often mild, subclinical and asymptomatic. [7] However, the infection may lead to herpetic gingivostomatitis, fever and pain after an incubation of approximately 1 week (2-20 days after contact). In healthy persons, sunlight or ultraviolet (UV) light are well-known trigger factors for RHL. Other common trigger factors are fever, common cold and other viral infections, in addition to physical trauma, including dental treatment and physiological factors such as elevated levels of stress, dietary inadequacy, menstruation or hormonal shifts in women [8] [9].
Diagnosis
Diagnosis of RHL is usually based upon the clinical history together with presented features. HSV-1 serology is the gold standard through antibody detection. To confirm an HSV-1 infection, detection of viral DNA by direct PCR (polymerase chain reaction) assays is a highly sensitive method of identifying appropriately infected cells that are not dependent on viable virus. [9]. The sample procedure is simple, noninvasive and requires only standard media for bacterial or virus samples. A biopsy may be indicated if lesions are atypical and/or remain chronic without a normal healing pattern.
Treatment
Avoiding direct contact of affected lesions is important to reduce the risk of spreading the virus. For instance, washing hands is important, as is not sharing, for example, a toothbrush or a glass. Herpetic whitlow, also known as herpetic paronychia, presents as a local pain or burning sensation followed by deep blisters that may secondarily erode, affecting the distal phalanx of one or more fingers [8] [9]. We mention it here as an occupational potential hazard for dentists to address the importance of using protective gloves during dental procedures. Ocular HSV infections may present with uni- or bilateral keratoconjunctivitis [8] [9]. Thus, dentists should wear protective glasses during patient procedures. Immunocompromised persons have a higher risk of RHL infection, longer duration of episodes, and are prone to comorbidities of HSV. For instance, viruses may spread to the central nervous system, causing encephalitis or meningitis [8].
The list of early preventive or management interventions for people prone to RHL is long [7], including sunscreen or protection of lips (lip balms/moisturizers, zinc-based creams, aloe vera gel, ice cubes, etc.). In the early prodromal phase, antiviral therapy is recommended (not later than 48 hours in this phase), as the drugs act through virus-statics by interrupting DNA replication. Numerous clinical trials conducted over the years still indicate that topical acyclovir 5% or penciclovir 1% remains the first-line therapy, being both effective and well tolerated [7] [8]. Systemic antiviral agents such as acyclovir are recommended in more severe cases. In patients with severe pain, local anaesthetics or painkillers may be used to reduce symptoms. Topical corticosteroids for the treatment of RHL lesions are contraindicated, as virus infection may progress and spread into the tissue and cause delayed healing.
Aphthous stomatitis
This oral mucosal condition can be divided into recurrent aphthous ulcers (RAS) and aphthous-like ulcerations (ALU) depending on whether the ulceration has a presumed etiology. RAS is an inflammatory condition of unknown aetiology characterized by painful, recurrent, single, or multiple ulcerations of mainly the nonkeratinized oral mucosa in otherwise healthy individuals [10]. These lesions often present in people aged 10-40 years, and a hereditary component may be present [11].
ALU, on the other hand, is an inflammatory condition characterized by painful, recurrent, single, or multiple ulcerations of mainly the nonkeratinized oral mucosa in patients with an underlying systemic disease, allergy, hematinic deficiency, or medication [12]. Clinically, these lesions cannot be distinguished from RAS; however, they often run a more complex clinical course requiring more extensive medical screening and management [13].
Clinical features
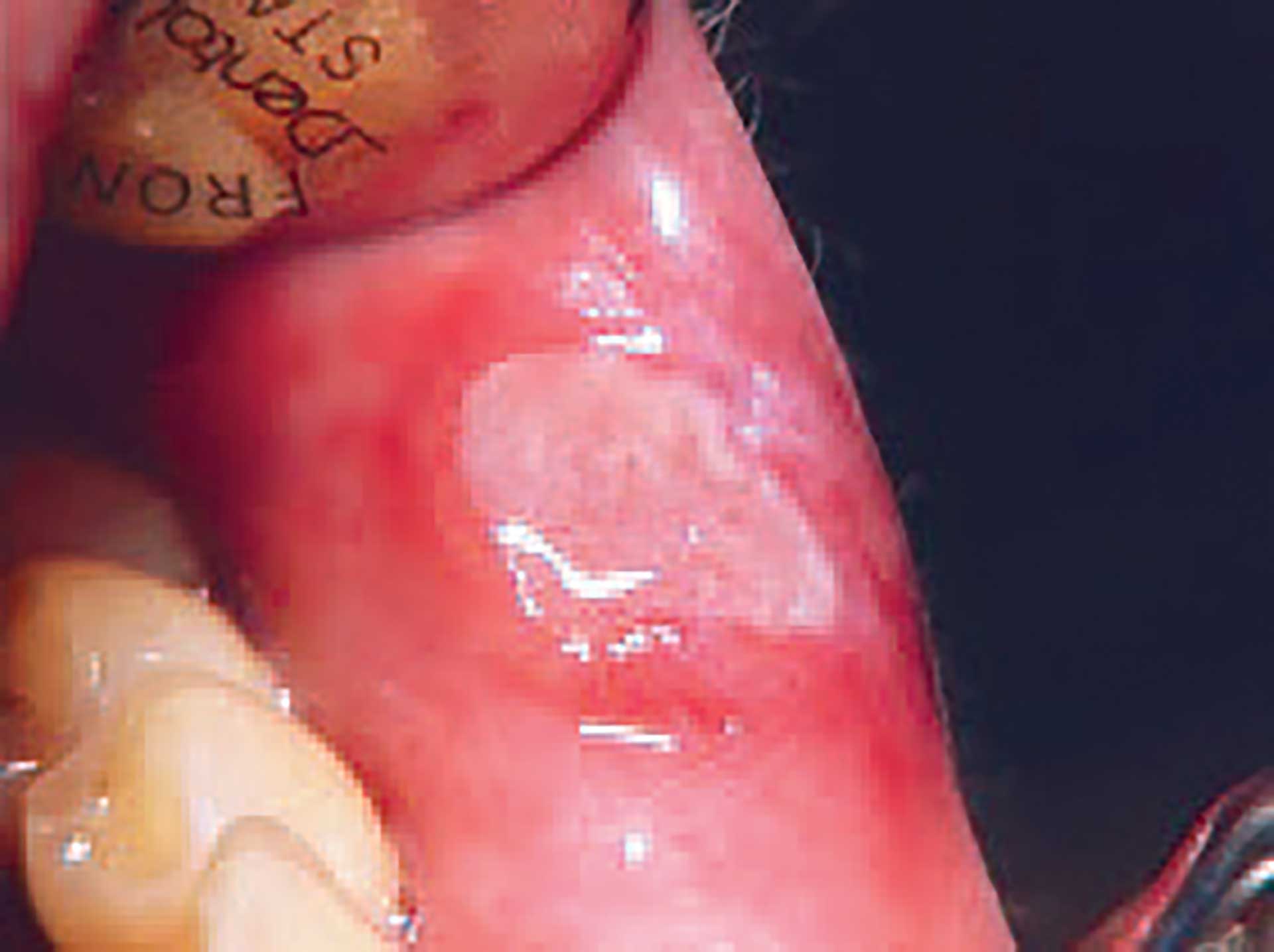
Figure 3. Aphtous ulcer on lip mucosa.
Aphthous ulcers present as well-demarcated round or ovoid lesions with a necrotic centre covered by a yellowish or greyish-white pseudomembrane encompassed by an erythematous halo, with the surrounding regions left clinically unaffected (figure 3). Ulcers of this kind are most commonly found on the labial and buccal surfaces. They may also present in the soft palate, ventral parts of the tongue, and floor of the mouth. They are rarely found on keratinized surfaces of the mouth, such as the gingiva, hard palate, or dorsum of the tongue. In addition, they are uncommonly found further down in the tonsils, uvula, and oropharynx, although when they are present in these areas, they are highly debilitating for the patient and more difficult to treat [13].
Patients with RAS are classically afebrile, without pallor, concomitant genital or ocular ulcerations and present with no history of immunodeficiency, whereas patients with ALU may display these features. The lesions can vary in size from 1 mm to over 10 mm in diameter, and heal within one week up to several weeks, sometimes with scarring.
Aetiology and pathogenesis
Most likely, the aetiology behind RAS is multifactorial, where many different factors contribute to the lesions that are typical of this condition [13]. Most patients are young and healthy but may have a hereditary component [14]. However, aphthous ulcerations can be associated with other factors, such as systemic diseases, medications, hematinic deficiencies and hypersensitivity to certain food substances [15]. Other factors that may influence aphthous ulcers are cessation of smoking, local trauma, dysbiosis of the oral microbiota, stress, salivary composition, toothpastes containing sodium lauryl sulfate (SLS) and hormonal changes [16].
Diagnosis
The diagnosis of RAS is mostly based on patient history, clinical examination and the exclusion of any underlying medical conditions or other contributory factors. There are currently no clinical diagnostic tests specific for RAS [17]. A biopsy is usually not necessary, as histopathological findings are not diagnostic and reveal only nonspecific ulceration. Sampling for viral, bacterial, or fungal infections can be required occasionally for differential diagnostic purposes. In the suspicion of ALU, a comprehensive medical history and clinical examination may guide the clinician through to a correct diagnosis. The most common systemic diseases include inflammatory bowel diseases, celiac disease, and Behcet’s syndrome.
Any extraoral complaint should be thoroughly evaluated, and if needed, a referral to the general practitioner or appropriate specialist should be considered. It is important to ask patients about any regular pattern of recurrence and accompanying fever. Other site involvement, such as skin, genital or gastrointestinal, can be ascertained through a formal systems enquiry. Laboratory investigations are useful to help exclude a potential systemic cause.
Treatment
At present, there is no cure for RAS. Instead, all the available treatment strategies are aimed primarily at relieving symptoms, such as pain, and secondarily with a focus on promoting healing and reducing the inflammation and risk of secondary infection. While various topical and systemic therapies have been used to treat RAS, there are few publications demonstrating the efficacies of the different substances applied, as stated in a recent meta-analysis [18]. Currently, there is no consensus available regarding appropriate treatment regimens for adults, and knowledge on the treatment of children barely exists [18].
Most patients have limited symptoms and do not require treatment. However, for those patients who do have symptoms, implying difficulties of eating and speaking, there are several options based on the simplest measure in relation to side effects and costs.
Pharmacological treatment:
Topical analgesics (for example benzydamine hydrochloride)
Systemic analgesics (for example, paracetamol, ibuprofen and naproxen)
Topical steroids (for example Triamcinolone and Clobetasol propionate)
Systemic steroids (for example, prednisolone)
Topical antibiotics (for example, chlortetracycline)
Other systemic treatments (for example, colchicine, pentoxifylline, dapsone, thalidomide, levamisole, montelukast, clofazimine and various biological therapies)
Nordic snus-associated oral lesions
In Nordic countries, snus primarily exists in four different forms: moist loose-weight snus (LWS), moist snus in pouches (SP), tobacco-free nicotine pouches (TfP) and tobacco- and nicotine-free snus in pouches (TNfS).
Clinical features
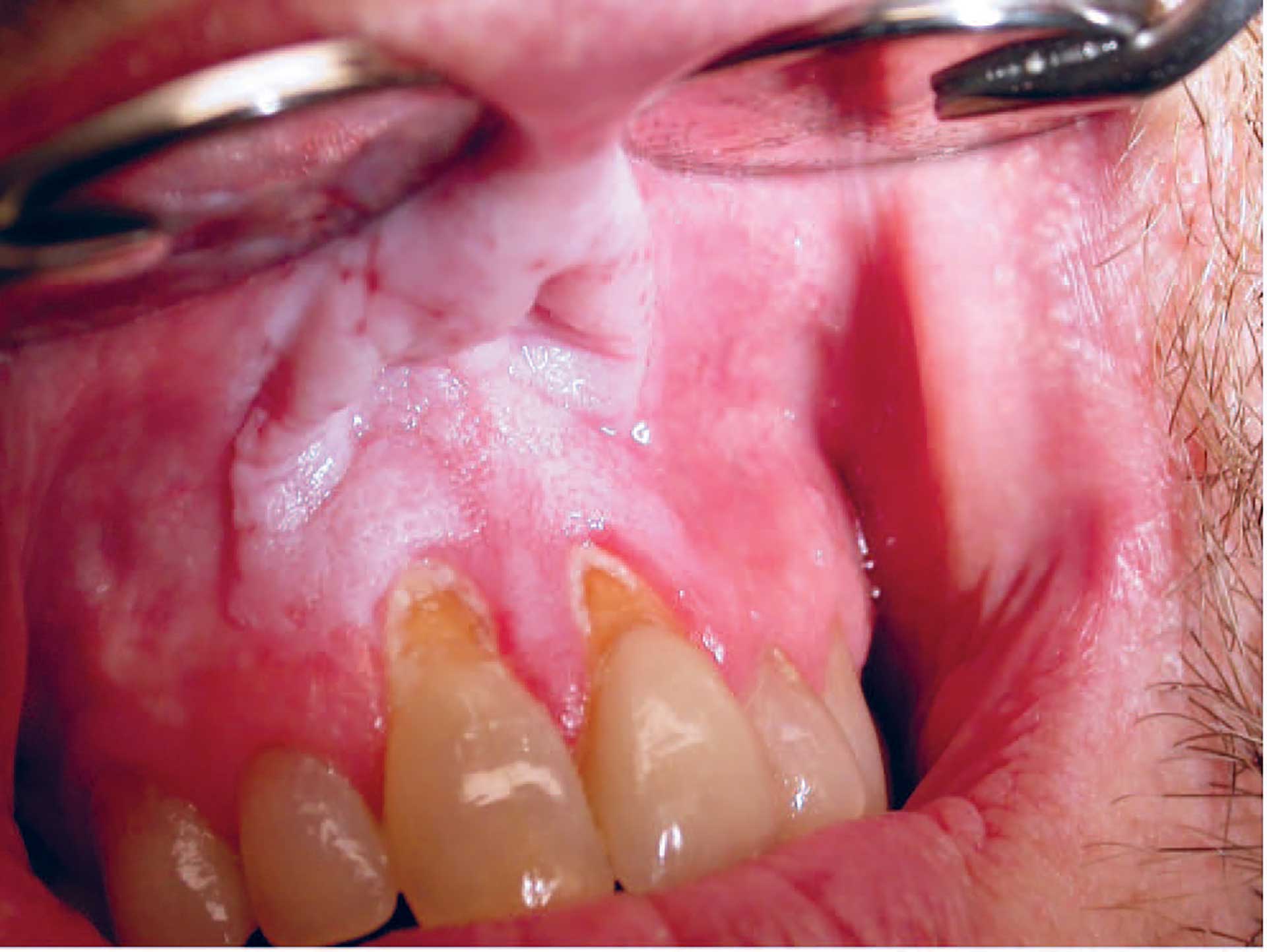
Figure 4. Snus-associated lesion.
The lesions are graded using four different stages according to the degree of severity [1]. The period of use and type of snus correlate to the degree of severity. The wrinkling and thickness of the oral mucosa (Fig. 4) increase in the following order: LWS/SP/TNfS. Gingival retractions have been observed in as many as 18% of the study individuals [19]. In the majority of cases, a completely normal mucous membrane is observed within a few weeks following cessation of snuff use. The cancer risk from snuff is extremely low, and epidemiological studies have not established causality. However, it cannot be completely ruled out that one or a few isolated cases may exist, most likely in individuals who have used loose snuff for a long time [20]. One must keep in mind that snuff other than Nordic snus, such as American or Asian snuff, bear a different risk for cancer.
Since its introduction in 2016, tobacco-free nicotine pouches (TfS) has become increasingly popular. In a recent study [21], 60 Swedish smokeless tobacco users were encouraged to substitute their snus with TfS during a 6-week period. Over time, a reduction in preexisting oral mucosal lesions was observed between baseline and the final visit. Although the scientific knowledge of oral mucosal lesions associated with TfS is very limited, an increase in the number of reported cases to SOMnet, a Swedish national electronic network for oral medicine, has been seen. These lesions are different from traditional oral mucosal lesions and have a more lichenoid nature. However, a more scientific evaluation is needed to ensure its existence.
Diagnosis
The topical relation to the pouch and the disease history leads to the diagnosis.
Oral leukoplakia
It is well known that carcinoma of the oral mucosa is sometimes preceded by a whitish or reddish lesion. This is why such lesions have been identified as potential precursors of cancer, now often termed ‘oral potentially malignant disorders’ (OPMDs), previously usually denoted premalignant lesions (PL), which we consider a more correct term. In fact, most patients diagnosed with PL do not develop oral carcinoma, but the lesions are associated with an increased susceptibility to cancer development [23]. The most important PLs in Scandinavia are leukoplakia and erythroplakia, the latter being extremely rare.
Clinical features
Leukoplakia can involve any site of the oral mucosa and may be uni- or multifocal and show a range of clinical features [24]. Homogeneous leukoplakia is characterized by a whitish, sometimes corrugated surface (figure 5), while nonhomogeneous leukoplakia shows a surface with white or red areas, often with nodules or seldom with verrucous excrescences. Leukoplakia has been described with a prevalence of 1% to 5% [19] [25] [26].
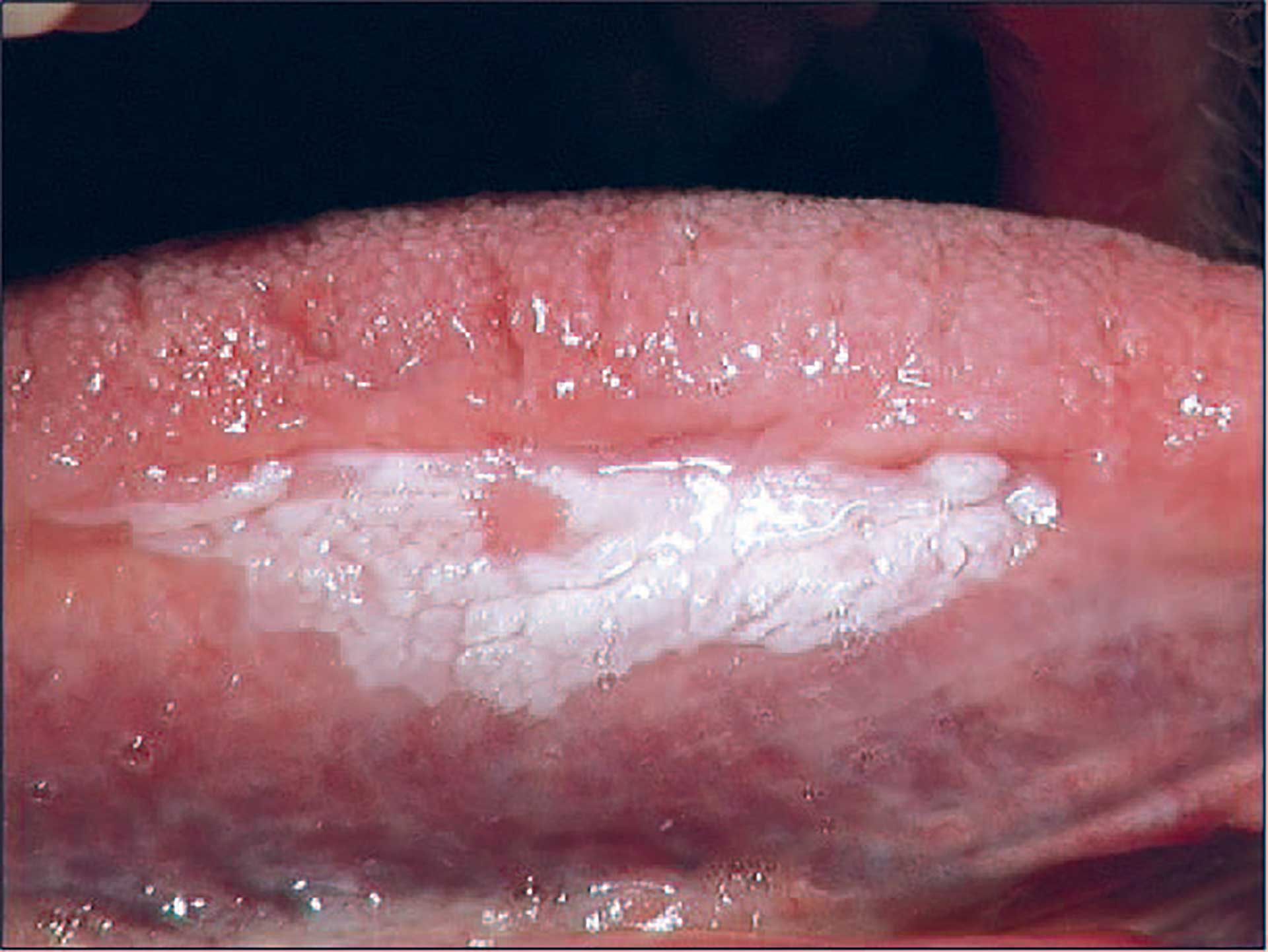
Figure 5. Oral leukoplakia.
Aetiology and pathogenesis
Although it has been the intention for a couple of decades to exclude lesions caused by tobacco from the diagnosis of leukoplakia, several problems are associated with this practice. Firstly, it is difficult to quit smoking and thereby explore the causal role of tobacco. Secondly, in smokers, the etiological role of tobacco is unknown. Thirdly, in many countries, including those in the Far East, a causal role of various smoking habits for leukoplakia has been regarded as a factor. Furthermore, virus ([27] [28] and yeast infections [29] have been suggested to be involved in the etiology of leukoplakia. Thus, Candida infection has been described in as many as 27-81% of cases [30]. However, there is no doubt that some oral leukoplakias have an unknown cause [25]. A study in private dental practice in the UK showed that heavy smokers were more likely to present with a leukoplakia or oral lichen planus by a factor of 3.58 for females and 3.68 for males [31]. This study also showed that heavily drinking men were approximately three times more prone to have such a lesion and that the prevalence increased with long-term use of tobacco and alcohol. In addition, a study carried out among the Bangladeshi population of East London showed a prevalence of leukoplakia of as high as 25%, with a positive association with betel quid chewing [32].
Studies of early stages of cancer development have resulted in the theory that cancer arises in areas with cellular genetic alterations associated with malignancy. Such so-called precancerous fields according to the theory of field cancerization may remain invisible up to the point of malignant progression in patients with de novo tumors. However, a precancerous field may also manifest itself as a visible lesion, such as leukoplakia. [33] [34] [35] [36] [37]. The invisible changes in the border zones of leukoplakia may explain why surgical removal of the lesions appears to have a low success rate [38].
Diagnosis
The patient’s history and clinical examination as well as a biopsy should exclude other known diseases. Lesions that do not fulfill the diagnosis of leukoplakia are affections that are reversible after elimination of traumatic influences on the mucosa, e.g., masticatory friction, friction from exaggerated use of a toothbrush and/or a sharp tooth that chafes the tongue during chewing.
Treatment
The basic concept of handling oral leukoplakia is to prevent malignant transformation, but currently, no universally approved therapy regimen has been established to distinguish which lesions will transform into a carcinoma [39]. As mentioned above, the reduction of tobacco consumption is important but a difficult task for patients. Surgical removal of the lesions has been shown not to eliminate malignant development, which is why patients should be continuously followed independently of surgical removal. However, since unrevealed carcinomas have been identified after serial sectioning of surgically removed lesions, excision may be recommended for diagnostic purposes. It may be advisable to refer patients with high-risk leukoplakia to specialist clinics for diagnostic investigation and follow-up [40]. In addition, it is important to note that nonhomogenous leukoplakias are frequently infected by Candida species and, if so, should be treated with antimycotics, which may result in changes in clinical appearance [41].
Prognosis
Between 0.3 and 17.5% of oral leukoplakia cases may be associated with the development of oral cancer [39] [42] [43] [44]. However, some leukoplakias may remain unchanged throughout the patient’s lifetime, and others even disappear spontaneously [38]. A significant reduction in tobacco consumption has resulted in a decrease in size or the disappearance of many lesions [45]. In general, nonhomogeneous leukoplakias carry a higher risk of malignant transformation than homogeneous leukoplakias [38] [46] [47]. A long-term follow-up study showed a seven times increased risk for nonhomogenous types compared with homogenous types and a 5.4 times increased risk for lesions exceeding 200 mm2 [38] [48]. While the risk of malignant transformation of oral leukoplakias in some studies has been related to the location (Kramer et al., 1978), other studies have not found site-associated differences in the risk of future malignant development [38] [49].
The histological demonstration of epithelial dysplasia in a biopsy in several studies appears to be related to an increased risk of malignant development [50] [49] [51] [52] [36], yet other studies have questioned such a relationship [38, 53, 54), and it is remarkable that 40% of cancers arise from biopsied leukoplakias without dysplasia. Part of the explanation might be that biopsies of leukoplakias may not be representative of the most advanced dysplastic changes in the lesion or that cellular genetic alterations may not be envisioned as dysplastic changes in all cases [43][55] [56]. Taken together, the finding of epithelial dysplasia should be regarded as a sign of concern, but the lack of epithelial dysplasia is no guarantee of a course without risk of malignant development.
Lichenoid disorders
Lichenoid disorders comprise several clinical and histologically similar affections of the oral mucosa, including lichen planus, which is an inflammatory disease of skin and mucosa, lichenoid contact lesions, e.g., in relation to dental fillings, lichenoid drug reactions, and graft-versus-host disease. It has been proposed that rather than distinguishing between several conditions, the collective term “oral lichenoid disease” should be used until further knowledge is established [23].
Clinical features
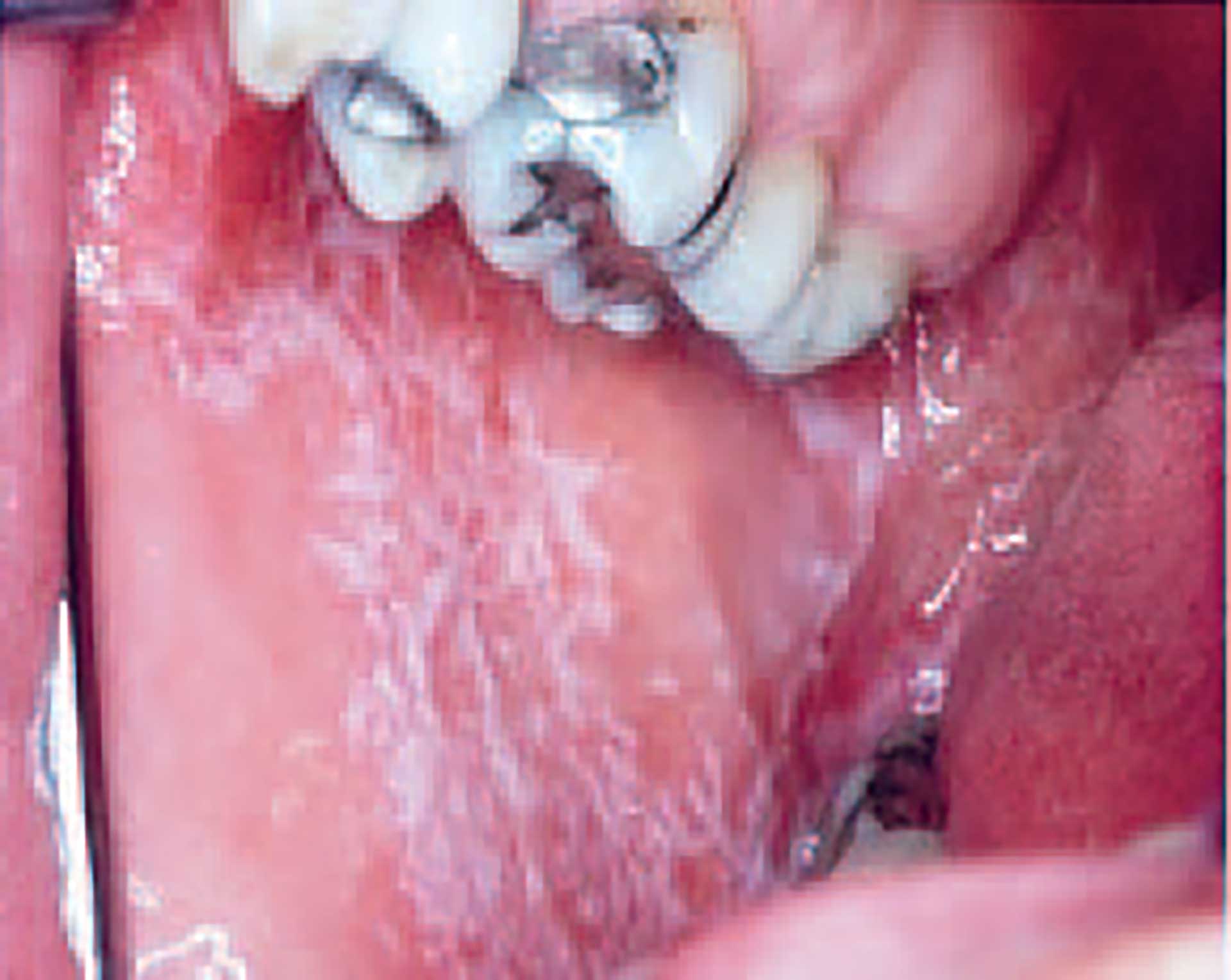
Figure 6. Lichenoid lesion on buccal mucosa.
The characteristic clinical picture of lichenoid disorders is the appearance of whitish streaks and papules (figure 6), often on a reddish background, but more uncharacteristic lesions, such as diffuse atrophic, reddish or whitish plaque-like changes, are usual, and sometimes ulcers appear [57]. If untreated, it is common for the lesions to persist for many years, but some lesions may change their clinical appearance. Thus, after many years, plaque-type lesions often appear, and sometimes it is difficult to distinguish such lesions from leukoplakia, especially since the typical lichen changes often disappear with time [57].
The lichenoid contact lesions are limited to the area of contact with the triggering dental material and therefore are usually unilateral as opposed to the other types of lichenoid lesions [58]. Oral lichenoid reactions in graft-versus-host disease (GVHD) are a complication that occurs in recipients of allogeneic hematopoietic stem cells or bone marrow.
The WHO has characterized oral lichen planus and oral lichenoid reactions as potentially malignant conditions [8] [9]. In patients with oral lichen planus, an increased development of oral cancer, i.e., in the order of 0.5-1.5%, has been observed in Denmark and Sweden [59] [60].
Aetiology and pathogenesis
The etiology of lichen planus is not established, and neither is any predisposing factor. Each of the other categories of lichenoid lesions are associated with an identified causal factor, e.g., treatment with filling materials for contact lesions [61], treatment with drugs for drug reactions and receipt of allogeneic hematopoietic stem cells or bone marrow for GVHD-associated lesions.
A striking histopathologic feature of oral lichenoid disorders is the subepithelial band-shaped inflammatory infiltrate dominated by lymphocytes characteristic of a type 4 hypersensitivity reaction, which also causes degenerative changes in the epithelial basal layer [62].
Diagnosis
Lichenoid contact reactions, drug-induced lichenoid reactions and the clinical manifestations of GVHD bear a striking resemblance to oral lichen planus both clinically and histopathologically. The diagnosis of drug-induced lichenoid reactions requires that a temporal connection can be demonstrated for the onset of the lesions and the administration of the suspected drug (e.g., antineoplastic and immunomodulating drugs) and whether the change disappears when the drug is discontinued. The clinical manifestations of GVHD alone are often sufficiently diagnostic, provided they are present in a patient who has received allogeneic hematopoietic stem cell transplantation. [63]
Treatment
In the case of symptoms linked to atrophic and ulcerating gingival lesions, it is important to carry out gentle but effective oral hygiene, which usually requires careful instruction of the patients. It is important not to brush directly on the soft tissue, as traumatic impact can worsen the lesions [64]. Secondary Candida infection of the lesions is common, and antimycotic treatment may sometimes resolve pain-associated ulcerative lesions. In cases of symptomatic lichenoid lesions on the inside of the upper lip in need of additional treatment, anti-inflammatory and anti-infectious therapy can be added, e.g., chlorhexidine 0.12–0.20% [65]. If necessary, it can be supplemented with antifungal treatment and topical steroid treatment. The treatment of contact reactions consists of replacing the triggering dental material, e.g., amalgam or composite, with, for example, gold or ceramics. GVHD usually requires systemic immunosuppressive treatment, including treatment with high-dose oral corticosteroids [66]. Patients should be followed by a regular dentist and only referred to a specialist dentist if there are specific problems, including if malignancy is suspected [67].
Geographic tongue
Clinical features
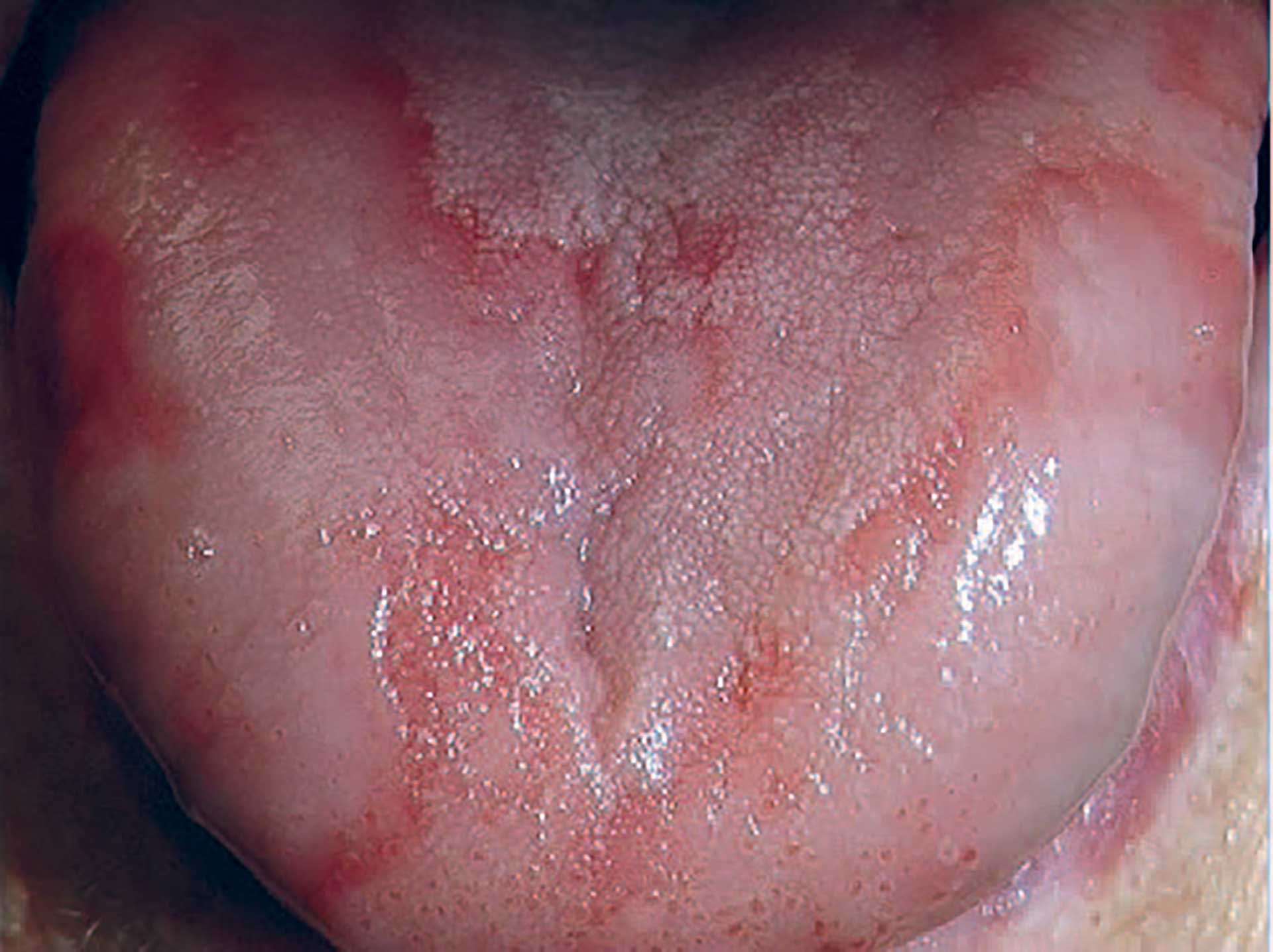
Figure 7. Geographic tongue.
The diagnosis of geographic tongue (GT) is primarily clinical, so it is fundamental to be acquainted with its clinical appearance. GT has several designations, such as benign migratory glossitis and erythema migrans. As the name suggests, this oral mucosal lesion is localized to the tongue but can also be found in other parts of the oral mucosa and is then called geographic stomatitis. GT is characterized by multiple well-defined areas with a depapillated erythema in the centre often surrounded by a slightly raised yellow‒white border in the periphery. [68]. The multiple lesions can sometimes form larger areas, giving the impression of a geographical appearance (figure 7). GT can disappear and reappear for periods of days, weeks, and months. In some cases, the GT lesion transforms into a fissured tongue. A recent meta-analysis of GT arrived at both the period prevalence and the point prevalence of 3.0% in the general population [69].
Most patients affected by GT do not report any discomfort. However, some patients complain of soreness, increased tongue sensitivity and burning sensations usually elicited by the intake of acidic foods and drinks.
Aetiology and pathogenesis
The etiology of GT remains largely unknown, but psychosomatic and hereditary factors have been proposed to play a role in the etiology of this mucosal lesion [70].
Diagnosis
GT rarely causes any major diagnostic problem. In certain cases, however, the clinical picture may differ, which justifies a biopsy. Inflammation characterizes the histology of the red depapillated areas of GT with loss of keratin and presence of neutrophil, lymphocyte and plasma cell infiltrates and intraepithelial microabscesses [71].
Treatment
Unfortunately, there is no well-established treatment for patients suffering from GT [72]. However, it is good to advise patients to reduce the intake of spicy, salty and acidic food, treat parafunctional habits, reduce stress levels, use products for dry mouth when necessary, eliminate the use of tongue scrapers and/or use local anasthetics such as lidocaine, xylocaine or benzydamin.
Pigmented lesions
Diagnosis of pigmented lesions of the oral cavity can be challenging. Clinical evaluation and assessment of differential diagnoses of the lesions should take into consideration the wide range of colours, from brown, black, grey, or blue, and the variation in number and extension. In addition, there may be overlapping clinical characteristics [73] [74] [75] [76] [77]. In general, a detailed clinical examination and medical history are important for the diagnosis.
Clinical features and etiology
A systematic overview of the various types of pigmentations is presented in Table 1. Clinically, pigmented lesions may present as focal (solitary), diffusely spread or as multiple oral and perioral spots. There may be clinical overlap between the different categories [73] [75] [77].
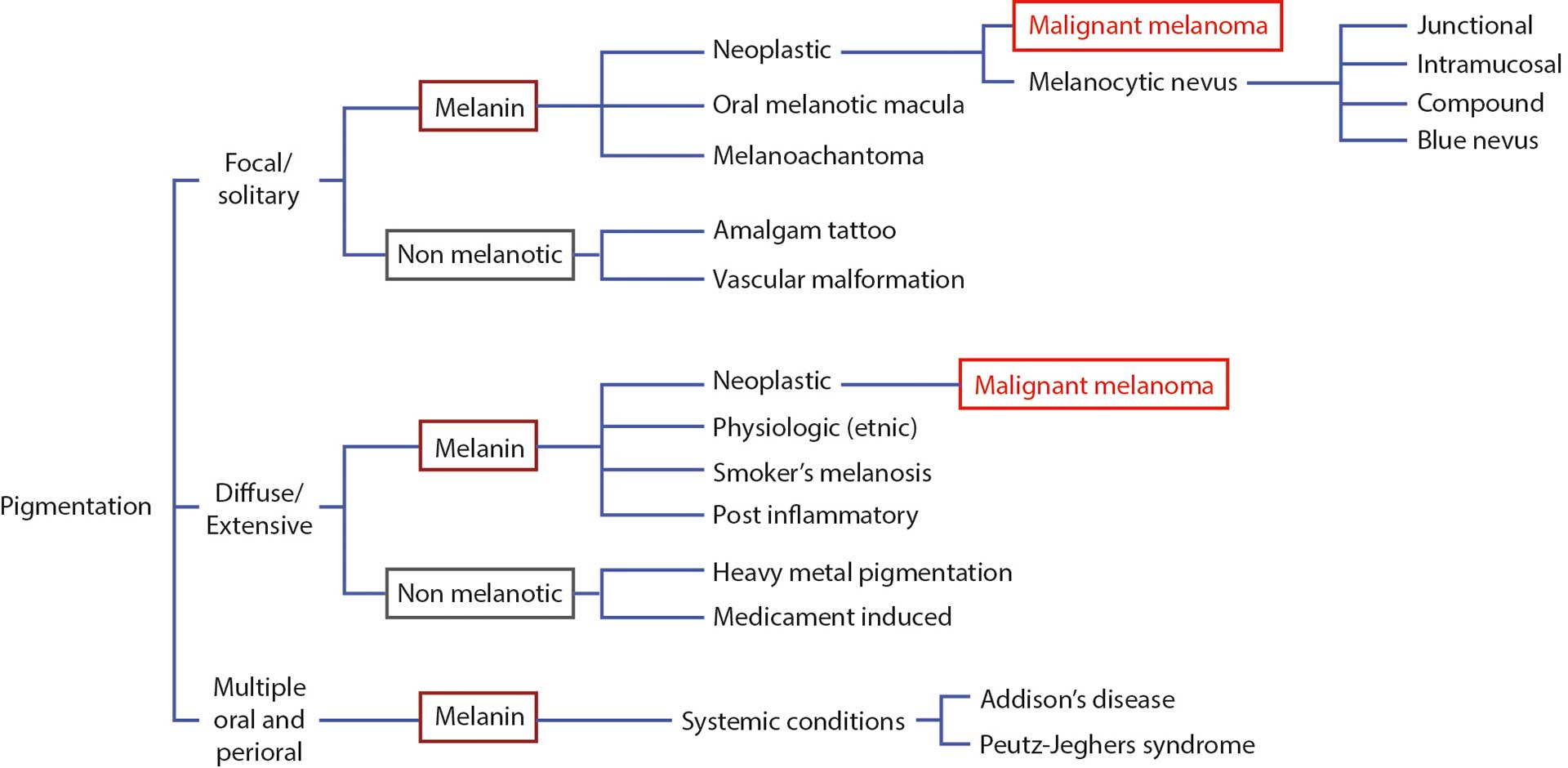
Table 1. A schematic overview of the various types of pigmentations.
Oral pigmentation may be physiologic, such as ethnic pigmentation. Such pigmentations are mostly diagnosed clinically. They manifest as diffuse or patchy symmetric brown pigmentations, most often in the gingiva. On the other hand, pigmentation may be the result of a pathologic condition, which requires a more careful analysis of the clinical picture.
Pigmentations can either be caused by melanin (located in melanocytes in the basal epithelial layer of the oral mucosa) or be nonmelanotic. The nonmelanotic group is rather diversified and includes exogeneous pigmentations, such as amalgam tattoos, heavy metal pigmentations, or medicament-induced pigmentations. The exogeneous pigmentations are most often grey or black and reflect metal depositions in the mucosa, such as silver or heavy metals (arsenic, bismuth, lead and mercury). [76]
The most common pigmented lesions of the oral maculas have been reported to be amalgam tattoos, oral melanotic macula and melanocytic nevus [73] [77]. Amalgam tattoos (focal argyrosis) manifest as grey or blue macules as a result of traumatic implantation of amalgam during dental treatment. They remain stable over time and in most cases without symptoms, since the foreign bodies are well tolerated by the body, provided they are implanted as small granules. Most often, they are located close to the teeth, preferably in the gingiva, but if, by accident, larger pieces of amalgam have been implanted in regions at a certain distance from the teeth, the diagnosis may not be obvious. In such cases, a foreign body reaction may have occurred, and lesions can be more extended.
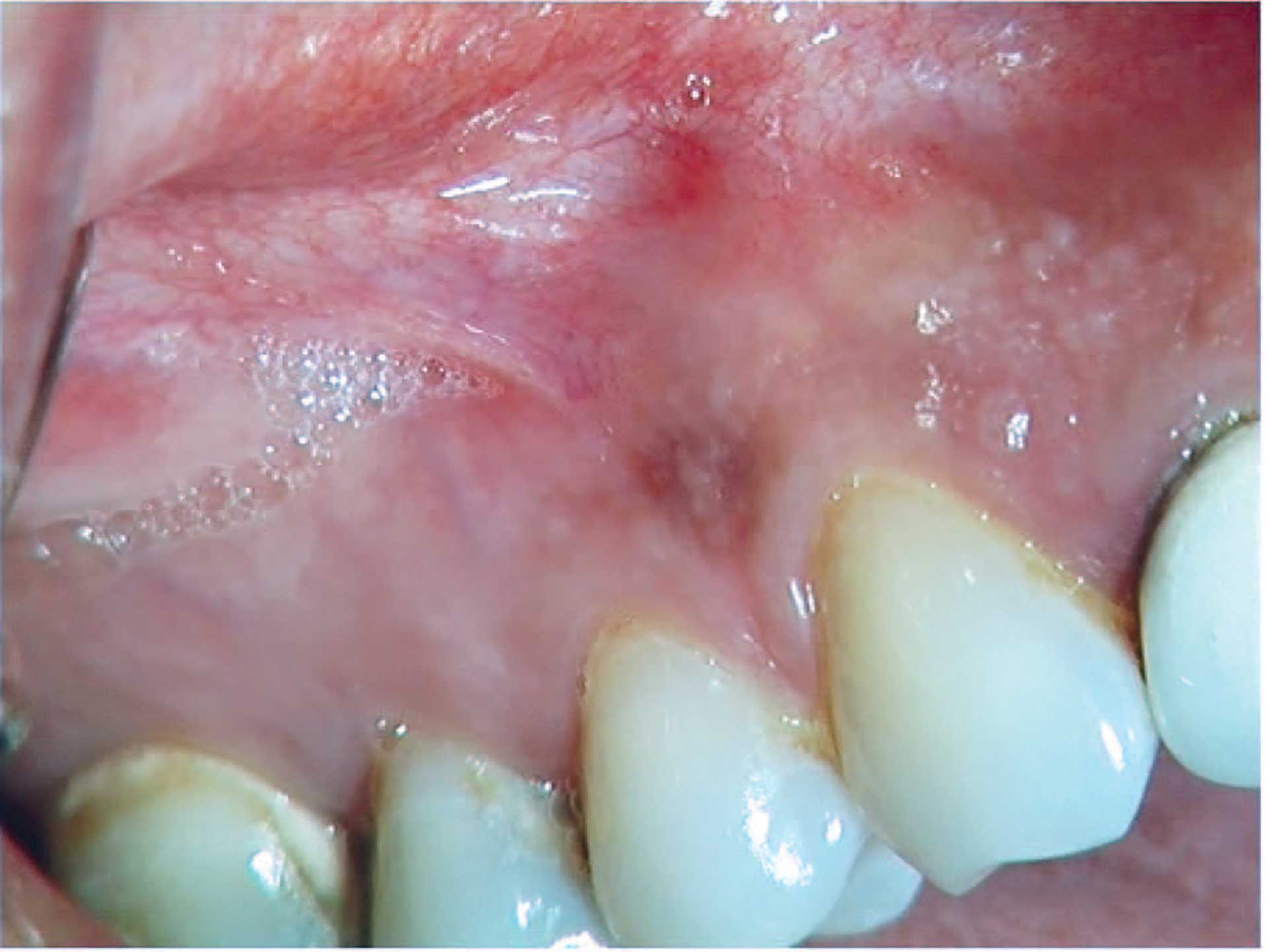
Figure 8. Melanotic macule.
The oral melanotic macule is a solitary homogeneous brown-to-black macule and is often <1 cm in diameter (figure 8). The macules are most often located in the gingiva and at the vermilion border, but they may occur at any location of the oral mucosa. Labial lesions are almost exclusively located on the lower lip. The pigmentation can be explained by increased production and deposition of melanin in the basal cell layer of the epithelium and in melanophages in the connective tissue [73] [77].
Intraoral melanocytic nevus may present as a macule or as an elevated papule or nodule <0.5 cm. It is most often located in the palate and less common in the buccal mucosa, labial mucosa, gingiva, alveolar ridge, and vermilion border. It is a benign tumor containing nevus cells. Histologically, four different subtypes are recognized (Table 1), depending on the location of the nevus cells. The most common type is intramucosal nevus, followed by blue nevus [73] [77]. There is no indication that an intraoral melanocytic nevus may undergo malignant transformation [74].
Diagnosis
Solitary pigmented lesions of the oral mucosa may mimic an early stage of malignant melanoma, which is extremely rare in the oral mucosa. Rapidly growing pigmented lesions should always be biopsied. Location in the palate increases the rate of suspicion of malignant melanoma. In skin, the ABCD checklist (asymmetry, border, irregularities, colour variation, and diameter > 6 mm) can be used in the diagnosis of cutaneous malignant melanoma. Such a checklist may also be used in the clinical diagnosis of oral melanoma. Histopathological diagnosis of pigmented lesions is important since there is an overlap in the clinical appearance of benign pigmented lesions and malignant melanoma [75]. Therefore, when in doubt, a biopsy must be performed to reveal the accurate diagnosis of this group of lesions [73] [74].
Pemphigus vulgaris
Clinical features
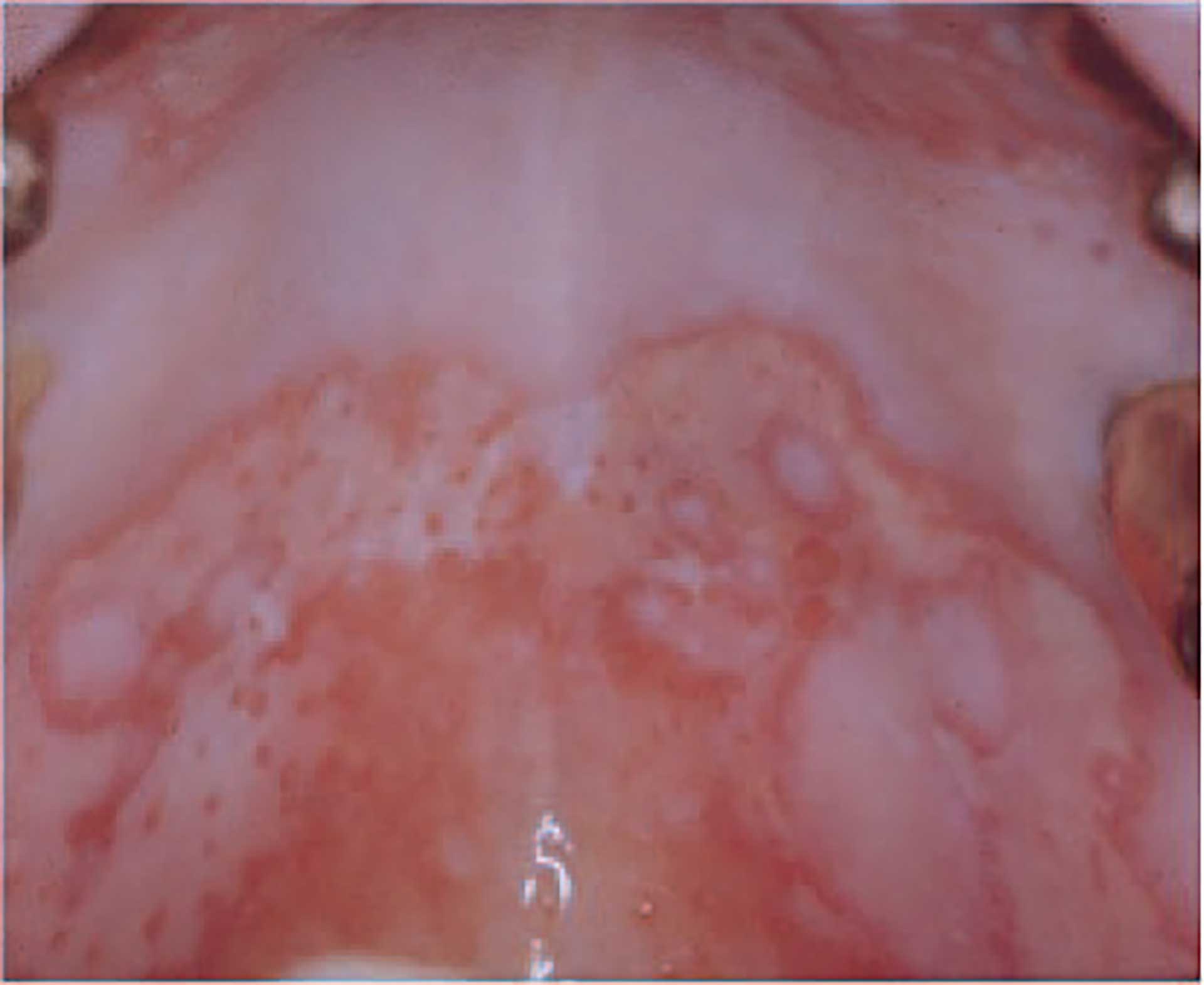
Figure 9. Pemphigus vulgaris.
Pemphigus vulgaris is an autoimmune bullous disease that typically involves the oral mucosa and skin. Due to autoantibodies against adhesion molecules on the surface of the epithelial cell lining of these tissues, intraepithelial bullae or erosions are formed (figure 9). Primarily due to secondary infections, the mortality rate is nearly 100% at the end of 5 years if left untreated. Although several effective treatment modalities are available, there may still be a 2-fold increase in the death rate compared with the general population. [78].
Pemphigus vulgaris occurs most frequently when patients are 50 to 60 years old, with an overall incidence that is slightly higher in females. Incidence estimates vary around the world according to geographic area and ethnic groups [78] [79]. The annual pemphigus incidence reported in northern Europe (Finland and Germany) is 0.5-0.98 per million, and in Iran, it is 16 per million, whereas the reported incidence for Israel and a Jewish population in the USA is 50 and 32 cases per million population, respectively [79] [80]. With increased immigration to Northern Europe, it is likely that the incidence of pemphigus vulgaris will increase in Scandinavia.
In nearly half of the patients, oral mucosal lesions develop before the skin lesions. Bullae that occur in the mouth are fragile and easily break, leaving irregular erosions, which are initially red but may later be ulcerated and covered by fibrin. Skin lesions usually appear 2-3 months later [79] [81].
Aetiology and pathogenesis
It was a breakthrough for the understanding of the disease mechanism and treatment possibilities when it was demonstrated that patients had antibodies to the epithelial cell membranes and that the amount of these antibodies correlated with disease activity. Later, it was shown that these antibodies are autoantibodies against the proteins desmoglein 1 and 3, which are adhesion molecules on the surface of epithelial cells. In most patients with disease localized to mucous membranes, only desmoglein 3 antibodies are found, whereas patients with mucocutaneous disease have antibodies against both desmoglein 1 and 3. These autoantibodies cause disease by the breakdown of cell adhesion [82]. Genetic factors have been identified as risk factors, although the correlation is unclear [79]. In some patients, environmental factors seem to play a role. For example, drugs such as penicillamine and captopril may interfere with the epithelium or the immune system and trigger an outbreak of pemphigus vulgaris [83].
Diagnosis
Histopathologically, pemphigus is characterized by lost adhesion of the epithelial cells in mucosa and skin, which is why the epithelium separates just above the basal epithelial cell layer, leaving the remaining epithelium as a single cell layer covering the connective tissue, clinically appearing as an erythematous lesion, denoted an erosion (figure 9). The diagnosis is based on clinical, histopathological, and immune pathological findings, often leaning on laboratory techniques from blood samples [82] [84].
Pemphigus vulgaris should be suspected in any patient with mucocutaneous erosions or bullae; histologically detected supra basal splitting of epithelium strongly supports this diagnosis, which may be further supported by demonstrating antibodies to epithelial cell membranes and/or to desmoglein [82] [84] [85].
Treatment
Physicians, usually dermatologists, take care of patients with pemphigus. Systemic corticosteroids administered at high daily doses have dramatically improved the prognosis, reducing mortality to <10%. However, after prolonged use, this is associated with significant side effects. Corticosteroid-sparing agents have been introduced to reduce the total dose of systemic corticosteroids. Other immunology-based treatment strategies have been introduced in recent years [81] [86]. Oral lesions may improve by minimizing irritations and by careful oral hygiene [86].
Mucous membrane pemphigoid
Mucous membrane pemphigoid (MMP) is a group of diseases characterized by bullous eruptions or ulcerations primarily at mucous membranes and concurrent autoantibodies to antigens in the basement membrane zone. MMP is a clinical phenotype in which subepithelial bullous formations may be induced by autoantibodies. It is a heterogeneous group of disorders with a chronic course and is primarily a disease of the mucous membranes, but occasionally the skin is involved as well. Previously, other names have been used to describe these conditions, including cicatricial pemphigoid, benign mucous membrane pemphigoid, oral pemphigoid, desquamative gingivitis, ocular cicatricial pemphigoid, and anti-laminin-5 cicatricial pemphigoid. Because it is difficult to clinically distinguish the various subgroups, the collective term mucous membrane pemphigoid is now accepted [87] [88].
Clinical features
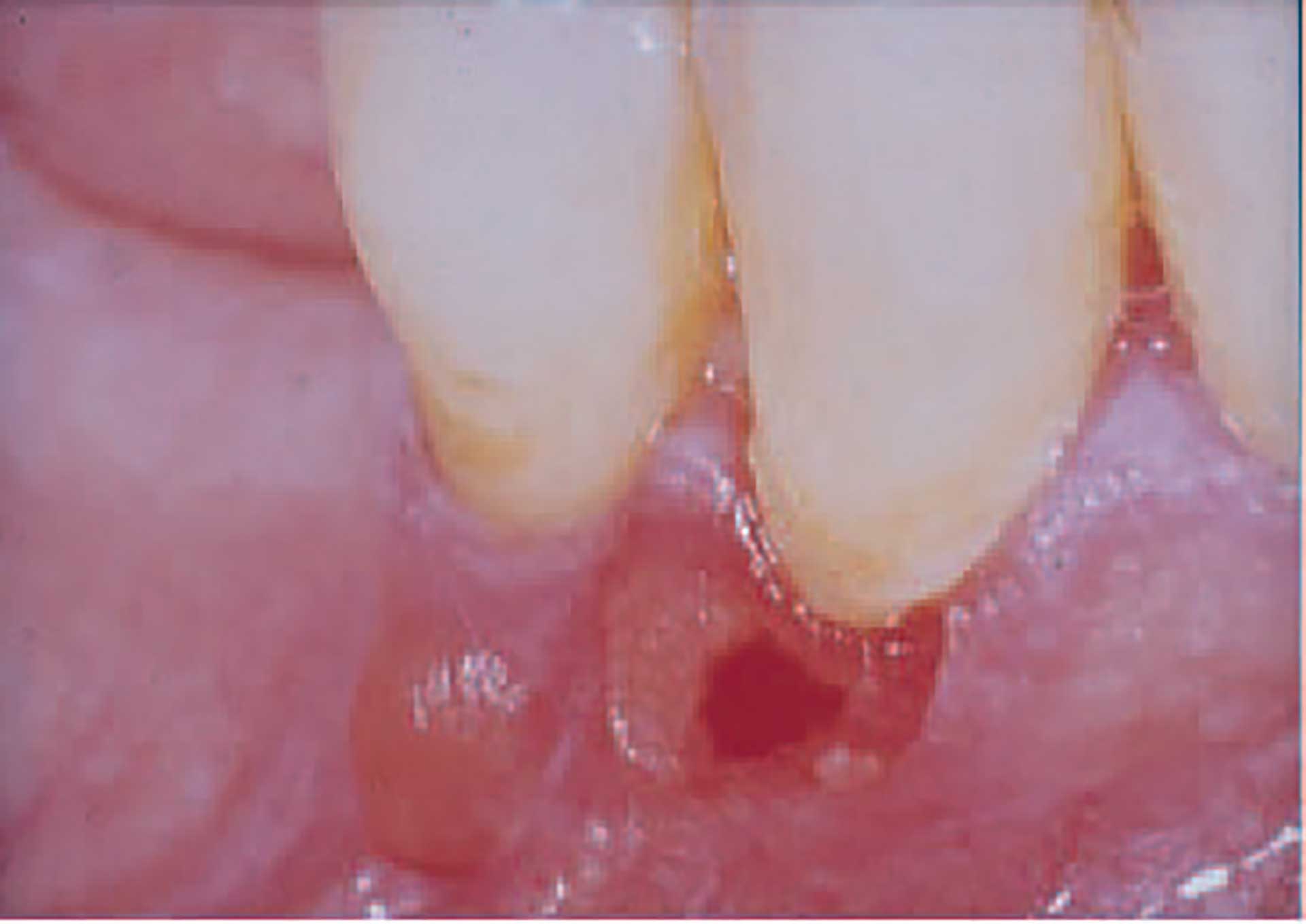
Figure 10. Bullae of mucous membrane pemphigoid.
The incidence of MMP is estimated at approximately 1.3 to 2.0 per million people. It characteristically presents in the 6th decade of life, and the female-to-male ratio is approximately 2:1 [89]. MMP most frequently affects the oral mucosa, but in 50% of patients, other mucous membranes (ocular, nasopharyngeal, laryngeal, esophageal, and genital) are also involved. Cutaneous involvement is usually absent or limited. The disease is severe with a varying prognosis, and healing of affections may produce irreversible scarring, except in the oral cavity. Thus, ocular affection may result in scarring with abnormal adhesion of the bulbar and palpebral conjunctiva (symblepharon), which may result in blindness [87] [90].
In the oral mucosa tense, sometimes hemorrhagic bullae develop; however, they often break due to trauma from normal functions, leaving ulcers covered by fibrin (figure 10). The lesions that may resemble late pemphigus lesions heal slowly. On attached gingiva, lesions may appear as diffuse reddening, often described as desquamative gingivitis. When lesions are biopsied, there is a tendency of the epithelium of the surrounding mucosa to loosen due to impaired adhesion of the epithelium to the underlying connective tissue.
Aetiology and pathogenesis
There is clinical and experimental evidence that autoantibodies to basement membrane proteins play a central role in the pathogenesis of the disease [87]. MMP autoantibodies may target different antigens in the basement membrane zone, and long-term follow-up studies indicate that the extent and severity of oral disease often correlate with antibody levels in patient serum [87] [91]. Genetics, including HLA alleles, which are a complex of genes encoding cell-surface proteins responsible for the regulation of the immune system, have been identified as risk factors [92]. It has been debated whether a minority of MMP patients have an increased relative risk of cancer; however, recent findings show that the rate of cancer in MMP patients does not differ from that of the general population [87].
Diagnosis
The diagnosis of MMP is based on clinical findings and detection of basement membrane zone autoantibodies. Basement membrane-bound antibodies can be identified by immunofluorescence staining of tissue sections from biopsies of lesions or surrounding mucosa, and other immunological techniques can detect circulating antibodies [93]. When repeated immunological investigations are negative, the diagnosis of MMP cannot be supported. [90] [91] [94]. Histopathologic features may be helpful but not conclusive; they are characterized by separation of the epithelium from the connective tissue at the level of the basement membrane zone [94].
Treatment
Due to the possible severe prognosis of extraoral MMP affections, it is important that the dentist pays attention to systemic manifestations when seeing patients with bullous lesions. Appropriate treatment and monitoring of MMP thus require a multidisciplinary team involving dermatology, dentistry, ophthalmology, and otorhinolaryngology. Topical corticosteroids are recommended as first-line therapy in mild/moderate oral MMP and as adjunctive therapy in moderate to severe oral MMP [95]. For gingival lesions, careful gentle oral hygiene often has a substantial positive effect by reducing the inflammatory burden of the periodontal tissues depending on the clinical distribution of lesions [96].
Fibroepithelial hyperplasia
Fibroepithelial hyperplasia is a reactive condition named after the clinical and histopathological presentation. It consists of fibroepithelial overgrowth because of injury or local irritation and is not a neoplasm. There is often an additional inflammatory component.
Clinical features
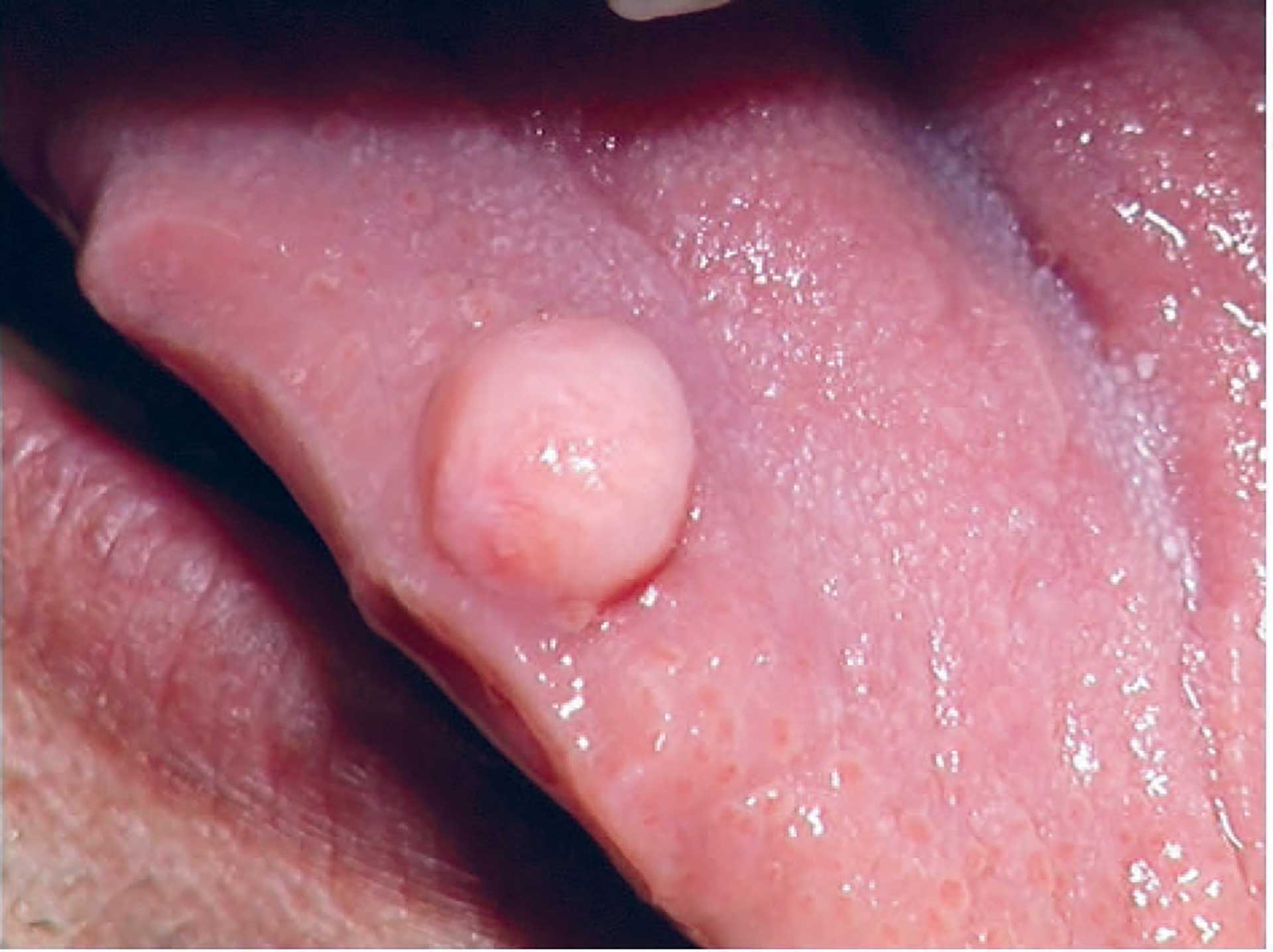
Figure 11. Fibroepithelial hyperplasia on tongue.
Clinically, these lesions present as a local, nodular growth (figure 11). The size is variable, but often they are rather small, less than 2 cm. They arise anywhere in the oral mucosa but most often on the buccal and lip mucosa as well as the gingiva and mobile tongue. They occur at any age but most often in 20-40-year-old adults with a female predominance. Fibroepithelial hyperplasias are often asymptomatic but may provoke discomfort or alter oral function, such as mastication or speech. They may also possess esthetic problems [97] [98].
Recurrence is uncommon and is mostly caused by repetitive trauma at the same site.
Aetiology and pathogenesis
Etiological factors consist of trauma or irritation, such as biting, fractured tooth, poor plaque control with calculus formation, local foreign body, or ill-fitting dentures. These factors cause mucosal irritation with a proliferative tissue response, often with some inflammation [97] [98].
Diagnosis
Fibroepithelial hyperplasias are a diagnostic challenge. It may mimic various pathologic processes, rarely even malignant lesions [99] [100]. Excision of the lesion is the choice of treatment, and there is a need for histopathological analysis to confirm the clinical diagnosis [100]. Histology shows fibroepithelial hyperplasia often with diffuse and mild lymphoplasmacytic inflammatory infiltration. The epithelium is parakeratinized and without dysplastic phenomena.
Treatment
Treatment is excision by surgery using local anaesthesia. Management should also always include the removal of irritative factors such as fixing the fractured tooth or ill-fitting prostheses or in case of poor hygiene and/or calculus, professional cleaning, and education of oral hygiene [99] [100].
References
Axell T. A prevalence study of oral mucosal lesions in an adult Swedish population. Odontol Revy Suppl. 1976; 36: 1-103.
Salonen L, Axell T, Hellden L. Occurrence of oral mucosal lesions, the influence of tobacco habits and an estimate of treatment time in an adult Swedish population. J Oral Pathol Med. 1990;19(4):170-6.
Robledo-Sierra J, Mattsson U, Svedensten T, Jontell M. The morbidity of oral mucosal lesions in an adult Swedish population. Med Oral Patol Oral Cir Bucal. 2013;18(5):e766-72.
Blagec T, Glavina A, Špiljak B, Bešlić I, Bulat V, Lugović-Mihić L. Cheilitis: A cross-sectional study-multiple factors involved in the aetiology and clinical features. Oral Dis. 2022; Aug 24, online ahead of print.
Ohman SC, Dahlen G, Moller A, Ohman A. Angular cheilitis: a clinical and microbial study. J Oral Pathol. 1986;15(4):213-7.
Cabras M, Gambino A, Broccoletti R, Lodi G, Arduino PG. Treatment of angular cheilitis: A narrative review and authors’ clinical experience. Oral Dis. 2019 Aug 29. doi: 10.1111/odi.13183. Epub ahead of print.
Gopinath D, Koe KH, Maharajan MK, Panda S. A Comprehensive Overview of Epidemiology, Pathogenesis and the Management of Herpes Labialis. Viruses. 2023;15(1):225.
Petti S, Lodi G. The controversial natural history of oral herpes simplex virus type 1 infection. Oral Dis. 2019;25(8):1850-65.
Arduino PG, Porter SR. Herpes Simplex Virus Type 1 infection: overview on relevant clinico-pathological features. J Oral Pathol Med. 2008; 37(2): 107-21.
Graykowski EA, Barile MF, Lee WB, Stanley HR, Jr. Recurrent aphthous stomatitis. Clinical, therapeutic, histopathologic, and hypersensitivity aspects. JAMA. 1966;196(7):637-44.
Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Häyrinen-Immonen R. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33(3):221-34.
Baccaglini L, Lalla RV, Bruce AJ, Sartori-Valinotti JC, Latortue MC, Carrozzo M, et al. Urban legends: recurrent aphthous stomatitis. Oral Dis. 2011;17(8):755-70.
Cui RZ, Bruce AJ, Rogers RS, 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34(4):475-81.
Miller MF, Garfunkel AA, Ram CA, Ship, II. The inheritance of recurrent aphthous stomatitis. Observations on susceptibility. Oral Surg Oral Med Oral Pathol. 1980;49(5):409-12.
Scully C, Porter S. Oral mucosal disease: recurrent aphthous stomatitis. Br J Oral Maxillofac Surg. 2008;46(3):198-206.
Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2014; 58(2): 281-97.
Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006;12(1):1-21.
Liu H, Tan L, Fu G, Chen L, Tan H. Efficacy of Topical Intervention for Recurrent Aphthous Stomatitis: A Network Meta-Analysis. Medicina (Kaunas). 2022;58(6).
Axell T, Mornstad H, Sundstrom B. The relation of the clinical picture to the histopathology of snuff dipper’s lesions in a Swedish population. J Oral Pathol. 1976;5(4):229-36.
Kopperud SE, Ansteinsson V, Mdala I, Becher R, Valen H. Oral lesions associated with daily use of snus, a moist smokeless tobacco product. A cross-sectional study among Norwegian adolescents. Acta Odontol Scand. 2023:81(6):473-478.
Hirsch JM, Wallström M, Carlsson AP, Sand L. Oral cancer in Swedish snuff dippers. Anticancer Res. 2012;32(8):3327-30.
Alizadehgharib S, Lehrkinder A, Alshabeeb A, Östberg AK, Lingström P. The effect of a non-tobacco-based nicotine pouch on mucosal lesions caused by Swedish smokeless tobacco (snus). Eur J Oral Sci. 2022; 130(4): e12885.
Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles M, Kerr AR, et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021;27(8):1862-80.
Warnakulasuriya S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020;102:104550.
Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37(1):1-10.
Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol. 2003;39(8):770-80.
Nielsen H, Norrild B, Vedtofte P, Praetorius F, Reibel J, Holmstrup P. Human papillomavirus in oral premalignant lesions. European journal of cancer Part B, Oral Oncol. 1996;32b(4):264-70.
Sundberg J, Korytowska M, Burgos PM, Blomgren J, Blomstrand L, Lara SDE, et al. Combined Testing of p16 Tumour-suppressor Protein and Human Papillomavirus in Patients With Oral Leukoplakia and Oral Squamous Cell Carcinoma. Anticancer Res. 2019;39(3):1293-300.
Krogh P, Hald B, Holmstrup P. Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis. 1987;8(10):1543-8.
Rindum JL, Stenderup A, Holmstrup P. Identification of Candida albicans types related to healthy and pathological oral mucosa. J Oral Pathol Med. 1994;23(9):406-12.
Lim K, Moles DR, Downer MC, Speight PM. Opportunistic screening for oral cancer and precancer in general dental practice: results of a demonstration study. Brit Dent J. 2003;194(9):497-502
Pearson N, Croucher R, Marcenes W, O’Farrell M. Prevalence of oral lesions among a sample of Bangladeshi medical users aged 40 years and over living in Tower Hamlets, UK. Int J Dent. 2001;51(1):30-4.
Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63(8):1727-30.
Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56(11):2488-92.85.
Partridge M, Pateromichelakis S, Phillips E, Emilion GG, A’Hern RP, Langdon JD. A case-control study confirms that microsatellite assay can identify patients at risk of developing oral squamous cell carcinoma within a field of cancerization. Cancer Res. 2000;60(14):3893-8.
Pindborg JJ, Daftary DK, Mehta FS. A follow-up study of sixty-one oral dysplastic precancerous lesions in Indian villagers. Oral Surg Oral Med Oral Pathol. 1977;43(3):383-90.
Poell JB, Wils LJ, Brink A, Dietrich R, Krieg C, Velleuer E, et al. Oral cancer prediction by noninvasive genetic screening. Int J Cancer. 2023;152(2):227-38.
Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long-term treatment outcome of oral premalignant lesions. Oral Oncol. 2006;42(5):461-74.
Holmstrup P, Dabelsteen E. Oral leukoplakia-to treat or not to treat. Oral Dis. 2016;22(6):494-7.
Holmstrup P. Can we prevent malignancy by treating premalignant lesions? Oral Oncol. 2009;45(7):549-50.
Krogh P, Holmstrup P, Thorn JJ, Vedtofte P, Pindborg JJ. Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol. 1987;63(1):48-54.
Pindborg JJ, Jolst O, Renstrup G, Roed-Petersen B. Studies in oral leukoplakia: a preliminary report on the period pervalence of malignant transformation in leukoplakia based on a follow-up study of 248 patients. J Am Dent Assoc. 1968;76(4):767-71.
Chaturvedi AK, Udaltsova N, Engels EA, Katzel JA, Yanik EL, Katki HA, et al. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. J Natl Cancer Inst. 2020;112(10):1047-54.
Reibel J. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14(1):47-62.
Roed-Petersen B. Effect on oral leukoplakia of reducing or ceasing tobacco smoking. Acta Derm Venereol. 1982;62(2):164-7.
Lind PO. Malignant transformation in oral leukoplakia. Scand J Dent Res. 1987;95(6):449-55.
Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):612-27.
Evren I, Brouns ER, Wils LJ, Poell JB, Peeters CFW, Brakenhoff RH, et al. Annual malignant transformation rate of oral leukoplakia remains consistent: A long-term follow-up study. Oral Oncol. 2020;110:105014.
Silverman S, Jr., Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53(3):563-8.
Kramer IR, El-Labban N, Lee KW. The clinical features and risk of malignant transformation in sublingual keratosis. Brit Dent J. 1978;144(6):171-80.
Cowan CG, Gregg TA, Napier SS, McKenna SM, Kee F. Potentially malignant oral lesions in northern Ireland: a 20-year population-based perspective of malignant transformation. Oral Dis. 2001;7(1):18-24.
Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(3):321-9.
Brouns VE, Stenveld HJ, Klomp GH, Brouns JJ. [Symptomatic treatment of lichen planus of the attached gingiva]. Ned Tijdschr Tandheelkd. 2014;121(10):489-92.
Fonseca-Silva T, Diniz MG, de Sousa SF, Gomez RS, Gomes CC. Association between histopathological features of dysplasia in oral leukoplakia and loss of heterozygosity. Histopathology. 2016;68(3):456-60.
Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Oral premalignant lesions: is a biopsy reliable? J Oral Pathol Med. 2007;36(5):262-6.
Thomson PJ, Wylie J. Interventional laser surgery: an effective surgical and diagnostic tool in oral precancer management. Int J Oral Maxillofac Surg. 2002;31(2):145-53.
Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A prospective follow-up study of 611 patients. J Oral Pathol. 1988;17(5):213-8.
Bolewska J, Holmstrup P, Møller-Madsen B, Kenrad B, Danscher G. Amalgam associated mercury accumulations in normal oral mucosa, oral mucosal lesions of lichen planus and contact lesions associated with amalgam. J Oral Pathol Med. 1990;19(1):39-42.
Holmstrup P, Thorn JJ, Rindum J, Pindborg JJ. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17(5):219-25.
Rodstrom PO, Jontell M, Mattsson U, Holmberg E. Cancer and oral lichen planus in a Swedish population. Oral Oncol. 2004;40(2):131-8.
Bolewska J, Hansen HJ, Holmstrup P, Pindborg JJ, Stangerup M. Oral mucosal lesions related to silver amalgam restorations. Oral Surg Oral Med Oral Pathol. 1990;70(1):55-8.
Holmstrup P, Soborg M. Cellular hypersensitivity to oral lichen planus lesions in vitro. Acta Allergol. 1977;32(5):304-15.
Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Mar;103 Suppl: S25.e1-12.
Holmstrup P, Schiøtz AW, Westergaard J. Effect of dental plaque control on gingival lichen planus. Oral Surg Oral Med Oral Pathol. 1990;69(5):585-90.
Backman K, Jontell M. Microbial-associated oral lichenoid reactions. Oral Dis. 2007;13(4):402-6.
Baumrin E, Loren AW, Falk SJ, Mays JW, Cowen EW. Chronic graft-versus-host disease. Part II: disease activity grading and therapeutic management. J Am Acad Dermatol. 2022 Dec 23 online ahead of print.
Mattsson U, Jontell M, Holmstrup P. Oral lichen planus and malignant transformation: is a recall of patients justified? Crit Rev Oral Biol Med. 2002;13(5):390-6.
Picciani B, Santos VC, Teixeira-Souza T, Izahias LM, Curty Á, Avelleira JC, et al. Investigation of the clinical features of geographic tongue: unveiling its relationship with oral psoriasis. Int J Dermatol. 2017;56(4):421-7.
Pereira R, de Oliveira JMD, Pauletto P, Munhoz EA, Silva Guerra EN, Massignan C, et al. Worldwide prevalence of geographic tongue in adults: A systematic review and meta-analysis. Oral diseases. 2022 Oct 8, online ahead of print.
Alikhani M, Khalighinejad N, Ghalaiani P, Khaleghi MA, Askari E, Gorsky M. Immunologic and psychologic parameters associated with geographic tongue. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014 Jul; 118(1): 68-71.
McNamara KK, Kalmar JR. Erythematous and Vascular Oral Mucosal Lesions: A Clinicopathologic Review of Red Entities. Head Neck Pathol. 2019 Mar;13(1):4-15.
González-Álvarez L, García-Pola MJ, Garcia-Martin JM. Geographic tongue: Predisposing factors, diagnosis and treatment. A systematic review. Rev Clin Esp (Barc). 2018;218(9):481-488.
Dhanuthai K, Theungtin N, Theungtin N, Thep-Akrapong P, Kintarak S, Klanrit P, et al. Pigmented Oral Lesions: A Multicenter Study. Eur J Dent. 2022;16(2):315-9.
Lambertini M, Patrizi A, Fanti PA, Melotti B, Caliceti U, Magnoni C, et al. Oral melanoma and other pigmentations: when to biopsy? J eur Acad Dermatol Venereol. 2018;32(2):209-14.
Meleti M, Vescovi P, Mooi WJ, van der Waal I. Pigmented lesions of the oral mucosa and perioral tissues: a flow-chart for the diagnosis and some recommendations for the management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(5):606-16.
Rosebush MS, Briody AN, Cordell KG. Black and Brown: Non-neoplastic Pigmentation of the Oral Mucosa. Head Neck Pathol. 2019;13(1):47-55.
Tavares TS, Da Costa AAS, Aguiar MCF, Loyola AM, Barcelos NS, Abreu M, et al. Differential diagnoses of solitary and multiple pigmented lesions of the oral mucosa: Evaluation of 905 specimens submitted to histopathological examination. Head Neck. 2021;43(12):3775-87.
Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch Dermatol Res. 2015; 307(4): 291-8.
Kasperkiewicz M, Ellebrecht CT, Takahashi H, Yamagami J, Zillikens D, Payne AS, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
Hietanen J, Salo OP. Pemphigus: an epidemiological study of patients treated in Finnish hospitals between 1969 and 1978. Acta Derm Venereol. 1982;62(6):491-6.
Pollmann R, Schmidt T, Eming R, Hertl M. Pemphigus: a Comprehensive Review on Pathogenesis, Clinical Presentation and Novel Therapeutic Approaches. Clin Rev Allergy Immunol. 2018;54(1):1-25.
Hammers CM, Stanley JR. Mechanisms of Disease: Pemphigus and Bullous Pemphigoid. Annu Rev Pathol. 2016;11:175-97.
Korman NJ, Eyre RW, Zone J, Stanley JR. Drug-induced pemphigus: autoantibodies directed against the pemphigus antigen complexes are present in penicillamine and captopril-induced pemphigus. J Invest Dermatol. 1991;96(2):273-6.
Murrell DF, Peña S, Joly P, Marinovic B, Hashimoto T, Diaz LA, et al. Diagnosis and management of pemphigus: Recommendations of an international panel of experts. J Am Acad Dermatol. 2020;82(3):575-585.e1.
Kershenovich R, Hodak E, Mimouni D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. 2014;13(4-5):477-81.
Scully C, Challacombe SJ. Pemphigus vulgaris: update on etiopathogenesis, oral manifestations, and management. Crit Rev Oral Biol Med. 2002;13(5):397-408.
Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370-9.
Lever WF. Pemphigus. Medicine (Baltimore). 1953;32(1):1-123.
Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol. 1995;131(1):48-52.
Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66(3):479-85.
Rashid KA, Gürcan HM, Ahmed AR. Antigen specificity in subsets of mucous membrane pemphigoid. J Invest Dermatol. 2006;126(12):2631-6.
Hesari R, Thibaut D, Schur N, Thoutireddy S, Witcher R, Julian E. Bullous Pemphigoid and Human Leukocyte Antigen (HLA)-DQA1: A Systematic Review. Cureus. 20233;15(6):e39923.
Bernard P, Antonicelli F, Bedane C, Joly P, Le Roux-Villet C, Duvert-Lehembre S, et al. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. 2013;149(5):533-40.
Schmidt E, Rashid H, Marzano AV, Lamberts A, Di Zenzo G, Diercks GFH, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part II. J Eur Acad Dermtol Venereol. 2021;35(10):1926-48.
Buonavoglia A, Leone P, Dammacco R, Di Lernia G, Petruzzi M, Bonamonte D, et al. Pemphigus and mucous membrane pemphigoid: An update from diagnosis to therapy. Autoimmun Rev. 2019;18(4):349-358.
Lee MS, Wakefield PE, Konzelman JL, Jr., James WD. Oral insertable prosthetic device as an aid in treating oral ulcers. Arch Dermatol. 1991;127(4):479-80.
Dutra KL, Longo L, Grando LJ, Rivero ERC. Incidence of reactive hyperplastic lesions in the oral cavity: a 10 year retrospective study in Santa Catarina, Brazil. Braz J Otorhinolaryngol. 2019 Jul-Aug;85(4):399-407.
Lakkam BD, Astekar M, Alam S, Sapra G, Agarwal A, Agarwal AM. Relative frequency of oral focal reactive overgrowths: An institutional retrospective study. J Oral Maxillofac Pathol. 2020 Jan-Apr;24(1):76-80.
Brierley DJ, Crane H, Hunter KD. Lumps and Bumps of the Gingiva: A Pathological Miscellany. Head Neck Pathol. 2019;13(1):103-113.
Vasanthi V, Divya B, Ramadoss R, Deena P, Annasamy RK, Rajkumar K. Quantification of inflammatory, angiogenic, and fibrous components of reactive oral lesions with an insight into the pathogenesis. J Oral Maxillofac Pathol. 2022;26(4):600.
Corresponding author: Mats Jontell, Department of Oral Medicine and Pathology, Institute of Odontology, Sahlgrenska Academy, University of Gothenburg, PO Box 450, 405 30 Göteborg, Sweden. E-mail: jontell@odontologi.gu.se
Accepted for publication 21.10.2023
The article is peer reviewed
The article should be cited as: Bankvall M, Dabelsteen E, Holmstrup P, Johannessen AC, Jontell M, Neppelberg E, et al. Common oral mucosal lesions. Nor Tannlegeforen Tid. 2024; 134: 124-36.
Keywords: diagnosis, mouth disease, oral manifestations, oral mucosa, oral medicine, oral pathology
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Bankvall M, Dabelsteen E, Holmstrup P, Johannessen AC, Jontell M, Neppelberg E, Rautava J. Common oral mucosal lesions. Nor Tannlegeforen Tid. 2024;134:126-38. doi:10.56373/2024-2-5
