Karin Garming Legert, Cecilia Larsson Wexell, Anne Marie Lynge Pedersen, Gita Gale, Victor Tollemar, Hellevi Ruokonen, Saara Kantola and Bente Brokstad Herlofson
Oral manifestations of systemic disorders – part 2
Associate professor, DDS, PhD. Division of Oral Diagnostic and Surgery. Department of Dental Medicine and Department of Orofacial Medicine – University Dental Clinic, Karolinska Institutet, Sweden
Associate professor, DDS, PhD. Senior consultant OMFS, Department of Oral and Maxillofacial Surgery, Skåne University Hospital, Lund, Department of Oral and Maxillofacial Surgery and Oral medicine, Faculty of Odontology, Malmö University and Department of Biomaterials, Sahlgrenska academy, University of Gothenburg, Sweden
Professor, DDS, PhD. Section of Oral Medicine and Pathology/Oral Biology and Immunopathology, Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
DDS, PhD. Department. of Oral Medicine and Pathology, Institute of Odontology, The Sahlgrenska Academy at University of Gothenburg, Sweden
Lecturer, DDS, PhD. Division of Oral Diagnostic and Surgey. Department of Dental Medicine, Karolinska Institutet, Sweden
Adjunct professor, DDS, PhD, eMBA. Oral and Maxillofacial Diseases and Surgery, Head and Neck Center, Helsinki University Hospital and University of Helsinki, Finland
Head, DDS, PhD, Dental Training Clinic, Wellbeing Services County of North Ostrobothnia, Oulu, Finland
Professor, DDS, PhD. Department of Oral Surgery and Oral Medicine, Faculty of Dentistry, University of Oslo, Norway, and Consultant, Unit of Oral Surgery and Maxillofacial Surgery, Department of Otorhinolaryngology – Head, Neck and Reconstructive Surgery, Oslo University Hospital, Norway.
Treatment- and medication-related side effects can affect the oral cavity and occur for both benign and malignant conditions. Immunocompromised individuals are predisposed to oral infections because the immune system plays a specialized role in host defense. Defects in specific functions of the immune system lead to increased susceptibility to specific pathogens. In this article, we address the impact of osteonecrosis of the jaw, osteoradionecrosis, treatment with monoclonal antibodies, chronic graft versus host disease, and viral infections. All these factors are of importance, especially in cancer treatment and/or allogeneic stem cell transplantation.
Treatment-related oral side effects and diseases
Osteonecrosis of the jaw (ONJ)
Osteonecrosis of the jaw (ONJ) is a rare but serious condition characterized by parts of the jawbone becoming necrotic and infected. The two most common causes of ONJ are related to antiresorptive medications, i.e., bisphosphonates, denosumab and antiangiogenic agents (designated medication-related osteonecrosis of the jaw, MRONJ), and radiotherapy involving the jawbone (designated osteoradionecrosis, ORN).
Medication-related osteonecrosis of the jaw (MRONJ)
The first reports of MRONJ were published in the beginning of 2000 after the introduction and approval of a new class of nitrogen-containing bisphosphonates for the prevention of bone complications in adults with advanced cancer [1][2]. Bisphosphonates are a class of inorganic drugs with various chemical structures. Bisphosphonates bind to the bone surface and accumulate in the bone where they are released by osteoclasts. In 2011, treatment with a monoclonal antibody, denosumab, was approved for the same indications [3]. Denosumab is an inhibitor of RANKL (receptor activator of nuclear factor kappa-B ligand) and inhibits the maturation of osteoclasts by binding to RANKL. In 2019, romosozumab (Evenity®), a humanized monoclonal antibody that targets sclerostin, was approved for postmenopausal osteoporosis [4]. Sclerostin is inhibited, which allows Wnt signalling in osteoblasts to promote bone formation and allows for the inhibition of RANKL.
Definition
The definition consists of three criteria: a) exposed bone or bone that can be probed through an intraoral or extraoral fistula (e) in the maxillofacial region that has persisted for more than eight weeks; b) current or previous treatment with antiresorptive therapy alone or in combination with immune modulators or antiangiogenic medication; and c) no history of radiation therapy to the jaws or obvious metastatic disease to the jaws [5].
Age and incidence
MRONJ affects adult patients at all ages treated with antiresorptive agents, bisphosphonates and denosumab. Postmenopausal women with primary breast cancer have also been shown to have a beneficial reduction in breast cancer recurrence and death from treatment with adjuvant bisphosphonate therapy, i.e., zoledronate 4 mg/ml every 6 months for 3–5 years [6]. The incidence rate of MRONJ in this group is unknown. A Scandinavian 5-year study followed 2900 patients with cancer who were treated with denosumab or zoledronate. MRONJ affects between 1.4% and 6.6% of patients [2]. The risk for developing MRONJ in patients treated with bisphosphonates and denosumab for osteoporosis indication and osteoporosis prophylaxis is very low [5].
Risk factors
Local and systemic risk factors (table 1) increase the risk for the development of MRONJ, and after 4 years of treatment, the risk is doubled [5]. For treatment with denosumab, there is a steep increase in the risk.
Risk Factors | ||
|---|---|---|
Treatment-related |
Patient-related |
|
Systemic |
Local |
|
Type of agent (nitrogen-containing BP, denosumab or/and anti-angiogenic agent), duration of treatment, cumulative dose, potency, route of adminstration (intravenous, subcutaneous, per oral, intramuscular), other medication (chemotherapy, steroids, thalidomide and biological agents |
Poor health status, chronic inflammatory disease, comorbidities, lifestyle (smoking, alcohol and high age) |
Odontogenic infections, dento-alveolar surgery (e.g. tooth removal), implant surgery, ill-fitting dentures, exostosis e.g. mandibular and palatal tori, poor dental health and poor oral hygiene |
Clinical features
MRONJ lesions present as areas of exposed bone, ranging from a few millimeters to larger areas involving a half jaw, often starting as a nonhealing alveolus following tooth extraction. In other cases, exposed bone occurs spontaneously, often lingually in the molar region of the mandible or related to a mandibular torus. The soft tissue and necrotic bone become infected, and the inflammatory reaction can vary from mild to severe. The symptoms can vary from mild to intense pain (figure 1) [5].
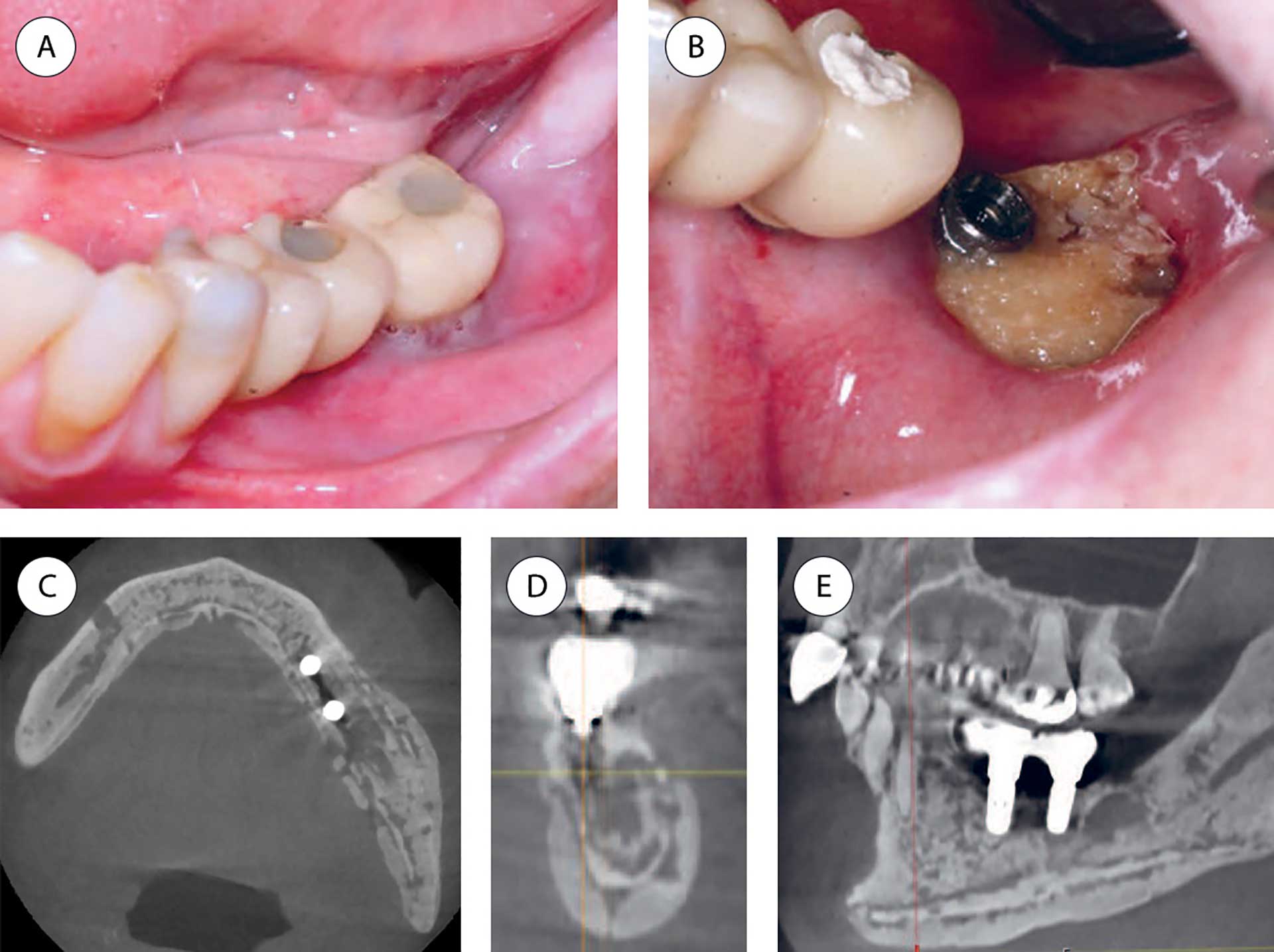
Figure 1 A-E. Patient with multiple myeloma treated with bisphosphonate (Zometa®) for 24 infusions during a 2-year period. Seven months after the last dose, the patient experienced pain related to the bone-anchored implants in the molar area. Both implants were installed several years before bisphosphonate treatment started. A: Before removal of the implant crown in the lower left second molar region. B: After removal of the implant crown in the lower left second molar region showing exposed necrotic bone. C-E: CBCT images demonstrate sclerosis and ostelytic areas involving a larger part of the mandible, including the body and ramus. The dental implants lost bone anchorage (axial, coronal and sagittal views).
Pathogenesis
The etiopathogenesis is still not clearly defined. Exposure to antiresorptive agents in combination with bone remodelling inhibition, inflammation and infection, angiogenesis inhibition, innate or acquired immune dysfunction, and genetic predisposition have been proposed as hypotheses [5].
Diagnosis
MRONJ is a clinical diagnosis. Panoramic X-ray can provide an overview and serve as a basis for confirming the diagnosis and determining whether further imaging techniques are needed. Cone-beam computed tomography (CBCT) or conventional CT, with and without contrast, are valuable techniques commonly used in more complex investigations to evaluate the extent of the disease. A biopsy of the affected area can contribute to support the clinical diagnosis, but the main indication for histopathological analysis of a lesion is to exclude malignancy.
Differential diagnostics
The most important differential diagnoses are secondary chronic osteomyelitis and primary malignant tumour or metastasis in the jawbone or surrounding tissues.
Treatment
The treatment of MRONJ may be nonsurgical or surgical. Nonsurgical treatment, often referred to as conservative treatment, includes maintenance of good oral hygiene and reduction of local soft tissue inflammation. Nonsurgical treatment may also include prolonged antibiotic therapy, similar to treatment for osteomyelitis. Surgical treatment ranges from sequestrectomy and block resections to segmental (continuity) resections and the insertion of reconstruction plates and is recommended for eligible patients.
Prognosis
Reports about the success rate of treatment of MRONJ often combine different MRONJ stages, underlying diseases, and drug treatments. In a recent review, extensive missing data and a lack of consensus were reported to be problems [7]. Optimal prevention of MRONJ includes regular dental check-ups, maintenance of good oral health and treatment of odontogenic infections and pathology in the jawbone and surrounding tissues before and during the treatment. It has been demonstrated that tooth extractions during/after antiresorptive therapy with low MRONJ incidences are possible provided that the following preventive measures are taken considering antibiotic therapy, alveoplasty, and primary wound closure [8].
Osteoradionecrosis (ORN)
In 1922, Regaud reported bone necrosis of the mandible after radiotherapy (RT) of cancer in the buccal mucosa [9]. Since then, numerous terms and definitions have been proposed for this feared and debilitating effect of RT.
Definition
The most frequently used definition of osteoradionecrosis (ORN) is exposed bone in a radiated field without healing over a period of 3 months without evidence of persisting or recurrent tumors [10][11]. However, some ORN cases appear to have necrotic bone (even to the extent of fracture) on radiology in the presence of intact skin and oral mucosa [12]. Radiology is thus an important tool for diagnosis and for describing the extent of the ORN condition.
Age and incidence
All patients treated with RT to the jaws run a life-long risk for developing ORN. The higher the amount of RT, the higher the risk for developing ORN. After the introduction of intensity-modulated RT (IMRT), the incidence of ORN was hypothesized to be reduced, but it persists with an incidence between 5-10% and arguably seems to increase, particularly in oropharyngeal cancer patients. The highest incidence of spontaneous ORN is seen in the first 2 or 3 years after RT, although cases have been reported more than 10 to 15 years after RT. Trauma-induced ORN (ORN secondary to, e.g., tooth extraction) has the highest incidence 2-5 years after RT [13].
Risk factors
The risk factors for ORN can be divided into treatment- and patient-related factors (table 2). Recently, an association between the development of ORN and the sites of pre-RT tooth extractions and other pre-RT-performed surgical procedures has been reported [14].
Risk Factors | ||
|---|---|---|
Treatment- related |
Patient-related |
|
Systemic |
Local |
|
Radiation dose > 55Grey, radiation field, radiation involving molars and teeth close to tumour, time to radio therapy < 14 days after removal of teeth especially molars i the mandible, chemotherapy |
Poor health status, older age, male gender, immune-deficiency, malnutrition, peripheral vascular disease, smoking, alcohol |
Site and size of tumour, poor oral hygiene, poor dental health and periodontal status, dry mouth, reduced mouth opening, ill-fitting dentures, dento-alveolar surgery (e.g. tooth removal), trauma to the oral tissues after radio therapy |
Clinical features
The clinical features of ORN are exposed bone, pain, swelling, trismus, oro-cutaneous fistula, and suppuration. Early radiographic features of ORN include widening of the periodontal ligament, coarsening of the trabecular pattern, osteolytic and sclerotic areas, and periosteal reaction, and further progression may show sequestration and pathological fracture (figure 2) [15]. These changes without any clinical features of ORN should not be diagnosed as ORN and require no treatment but should be regularly assessed since they could be a sign of early ORN.
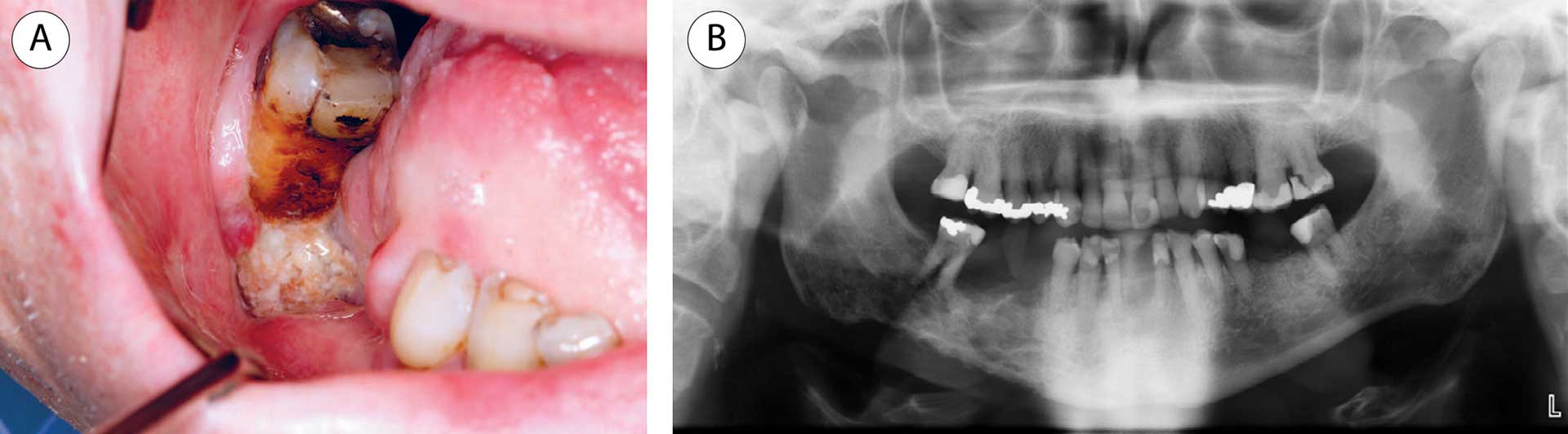
Figure 2 A-B. A: Patient with tongue cancer, treated twice with radiotherapy due to recurrence of the cancer, developed pain and discomfort in the right lower jaw 7 years after radiotherapy. The lower right first molar was endodontically treated and later removed. The patient developed bone necrosis with an exposed jawbone. B: An orthopantomogram displayed an osteolytic and irregular appearance in the mandibular area. Four years later and 13 years after radiotherapy, a pathological fracture occurred.
Pathogenesis
The cause of ORN is most likely multifactorial. The most accepted current theories are the hypoxia, hypocellular, and hypovascular theory (3H theory), followed by the radiation-induced fibrosis theory (RIF theory) [16][17].
Diagnosis
The diagnosis of ORN is based on the history of RT, clinical signs and symptoms and radiological findings. Several imaging techniques may be used for ORN detection, such as panoramic radiography, CT or cone beam CT (BCT), MRI, positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Panoramic radiography and CT/CBCT are most commonly used.
Differential diagnostics
Diseases and conditions that may show similar clinical and radiographical features as described for ORN include MRONJ (due to antiresorptives, antiangiogenics or immunosuppressants), bone infections (i.e., herpes zoster, tuberculosis or osteomyelitis), genetic diseases (i.e., sickle cell anemia), and environmental exposure (i.e., to phosphorous or radium).
Management and treatment
Oral and maxillofacial surgeons and head and neck surgeons take care of patients with ORN, often at the same hospital where the cancer treatment was performed. Currently, there are no consensus statements or guidelines for the management of ORN.
The treatment of ORN may be nonsurgical or surgical. Nonsurgical treatment, often referred to as conservative treatment, includes maintenance of good oral hygiene and reduction of local soft tissue inflammation primarily for symptom relief and prevention of disease progression. Nonsurgical treatment may also include longer periods of antibiotic treatment, corresponding to the protocol for osteomyelitis treatment. PENTO (PentoxifyllinTocoferol) or PENTOCLO (PentoxifyllinTocoferolClodronate) have been suggested as pharmacological treatments to influence red blood cells and inhibit the inflammatory response [17]. Infection control prior to and during such treatment is key to achieving a high rate of healing [18]. Surgical treatment ranges from sequestrectomy and block resections to segmental (continuity) resections and extensive surgical intervention, such as resection with or without vascularized free tissue flaps. Hyperbaric oxygen therapy (HBO) is still included in the protocol in some areas/countries when surgical treatment is indicated for ORN and when tooth removal after RT is needed.
Prognosis
Patients treated with radiotherapy for head and neck cancers have a life-long, and with time, increasing risk of developing ORN. Since tooth extraction in the irradiated jawbone is one of the most important risk factors for the development of ORN, this should be done at hospitals by specialists with knowledge in treating these patients.
Advanced therapy medicinal products (biologics)
Definition
A biopharmaceutical is any drug manufactured from a biological source made from complex molecules. Many biological drugs are produced using recombinant DNA technology. The European Medicines Agency (EMA) uses the term advanced therapy medicinal products (ATMP) for medicines for human use that are “based on genes, cells, or tissue engineering”, i.e., manufactured using living microorganisms, plants, human or animal cells [19].
Background
During the last decade, ATMPs have become a valuable contribution and a new opportunity for the treatment of different diseases [20][21]. They can be classified into three main types: 1) gene therapy medicines, 2) somatic-cell therapy medicines and 3) tissue-engineered medicines. Combined ATMPs can be both cells and a biodegradable matrix or scaffold (medical device). Examples of conditions treated with gene- and somatic-cell therapy medicines are cancer, rheumatoid arthritis and asthma [19][20][21].
Oral aspects
The oral side effects of ATMPs are poorly characterized, resulting in a lack of guidance for dental practitioners. Case reports describing oral side effects such as oral lichenoid reactions, oral mucosal ulcers and pigmentation are emerging [22][23]. Large multicenter studies will be necessary to further define the oral and dental complications caused by biologic agents [23]. The recommendations regarding oral health and accuracy when handling teeth and oral mucosa are the same as those for antiresorptive agents. The need for preoperative drug cessation varies (1 week - 4 months) and must be discussed with the responsible physician depending on the type of oral intervention. Treatment with ATMPs has been proposed as a possible medication for some oral conditions, i.e., JAK inhibitors for potential future treatment of oral lichen planus. Studies have demonstrated improvement for lichen planopilaris (a lichen affecting the nails) and erosive lichen planus, but the agents need to be further tested in clinical trials [24].
Oral chronic graft versus host disease
Definition
Graft versus host disease (GVHD) is a serious multiorgan complication in patients treated with allogeneic hematopoietic stem cell transplantation (HSCT). Patients receive donor cells that can immunologically respond to the host environment as a foreign body, leading to manifestations resembling an autoimmune syndrome such as GVHD [25][26]. GVHD can be acute or chronic based on clinical recognition, but acute (a) GVHD often occurs within the first 100 days post-HSCT. The main organs for aGVHD are the skin, liver, and upper and lower gastrointestinal (GI) tract, whereas oral aGVHD seldom occurs [27]. Chronic (c)GVHD often manifests in multiple organs, including the skin, mouth, liver, GI tract, lungs, eyes, and genitals [28]. Oral cGVHD features three disease presentations: lichenoid-like mucosal lesions, Sjögren’s syndrome-like sicca symptoms and limited mouth opening due to sclerosis.
Age and incidence
Children and adults may be treated with allogenic HSCT, but children do not develop GVHD to the same degree as adults, possibly due to restored thymus function. Nevertheless, GVHD remains a potential serious oral complication in the pediatric population. In adults, approximately 30-70% of patients surviving HSCT will develop GVHD. The oral cavity is frequently affected (45-83%) in patients with cGVHD [29][30].
Predisposing factors
Risks associated with GVHD development include donor and patient match, gender, age, virus serology and human leukocyte antigen, source of donor stem cells, preparative HSCT treatment regimen (radiotherapy and/or chemotherapy) and underlying disease [31]. Specific cGVHD risk factors include earlier episodes of aGVHD, mismatched donors and the use of peripheral blood stem cells (PBSCs) [32].
Clinical features
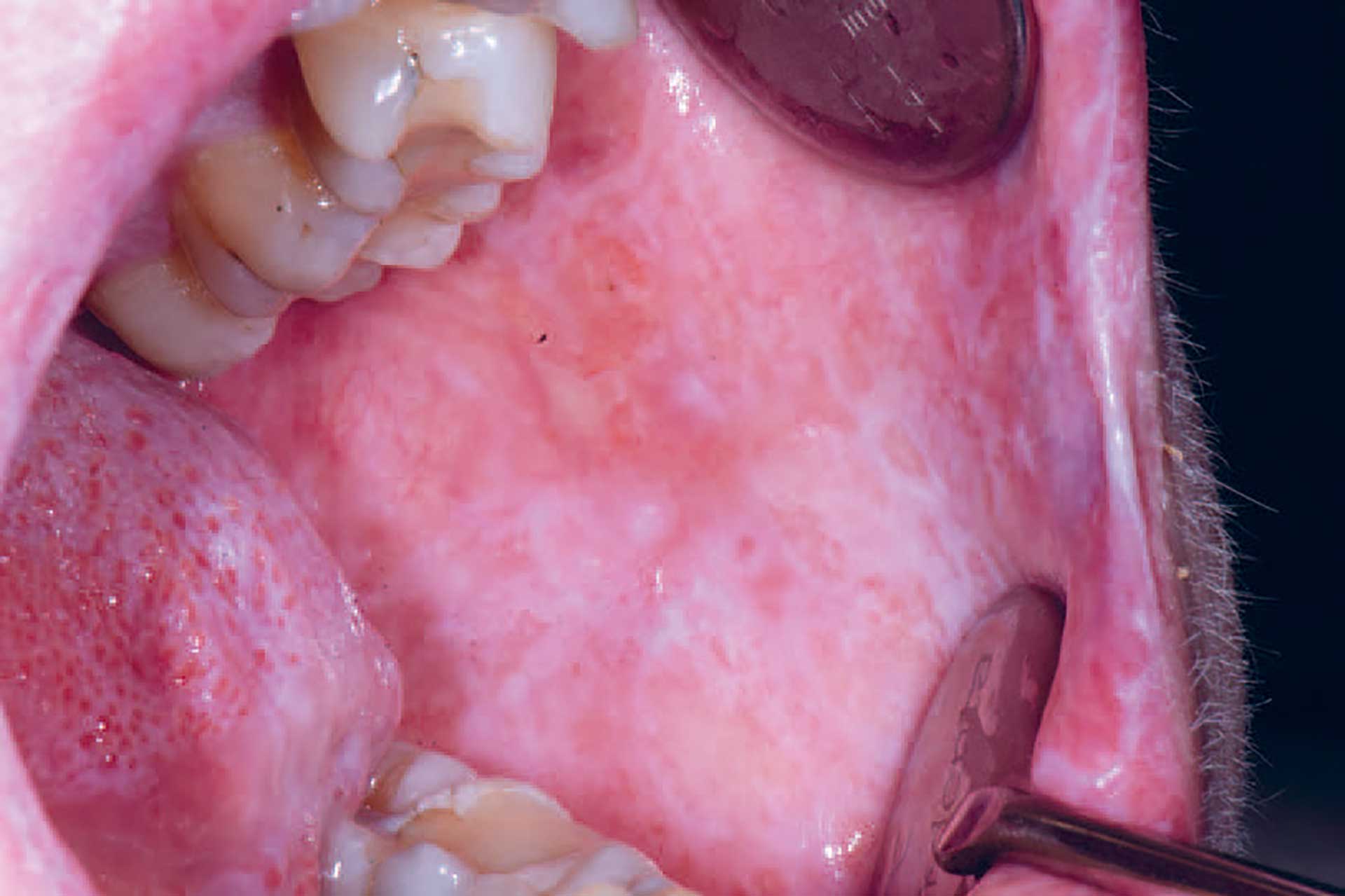
Figure 3. cGVHD in the buccal mucosa with lichenoid striations and ulcerations.
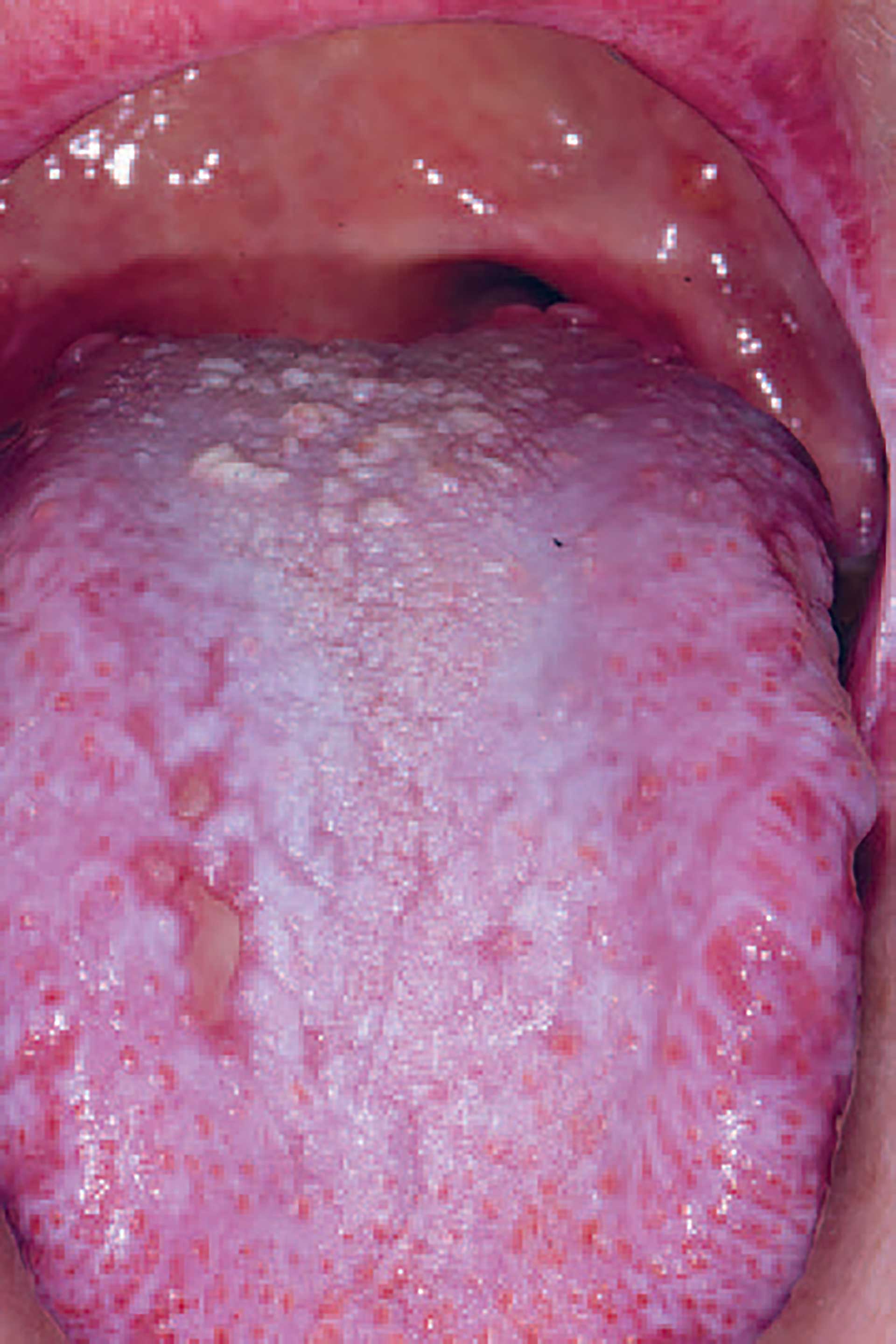
Figure 4. cGVHD on the dorsum of the tongue presenting with lichenoid striations, plaques and ulcerations.
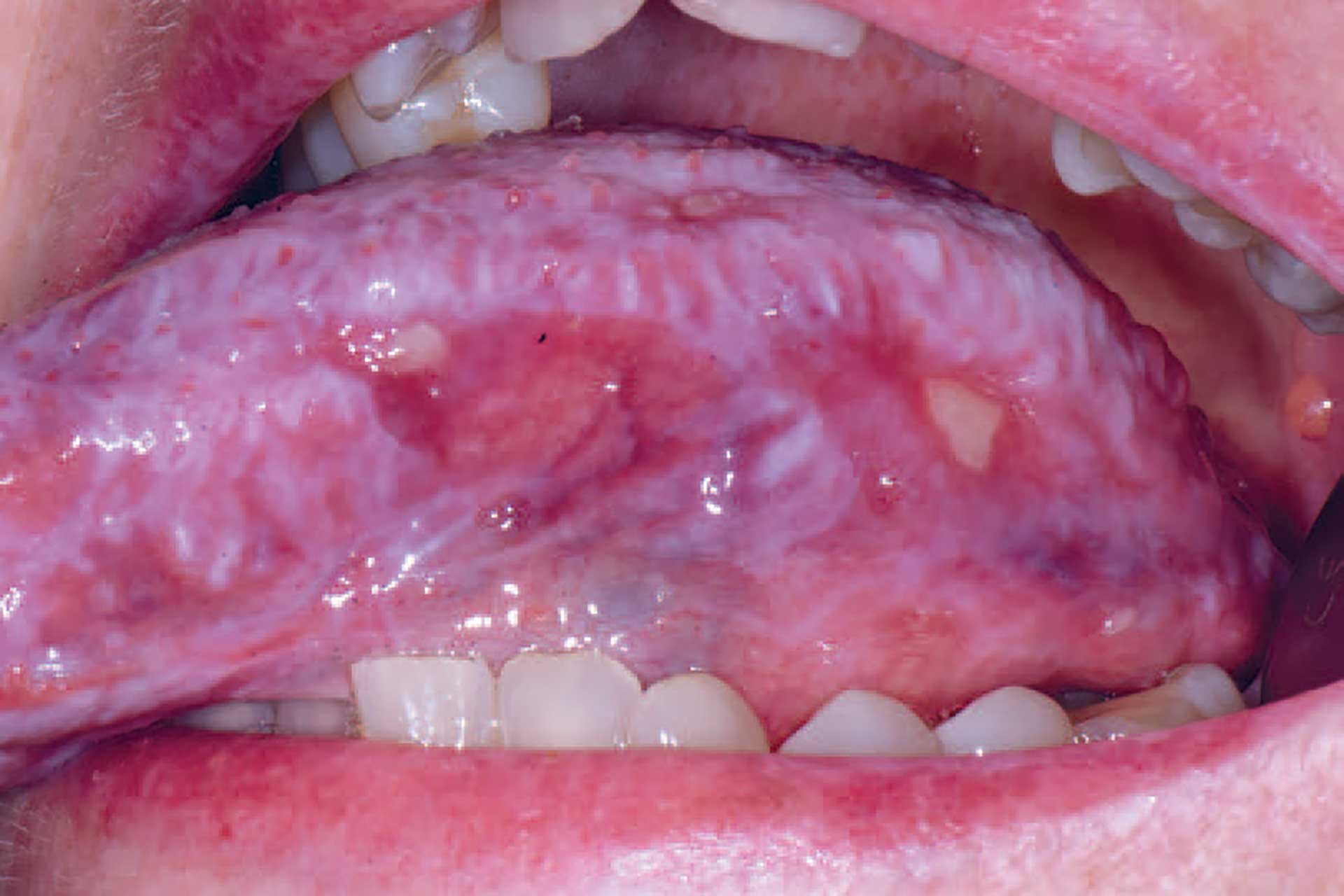
Figure 5. cGVHD on the tongue with lichenoid striations and ulcerations.
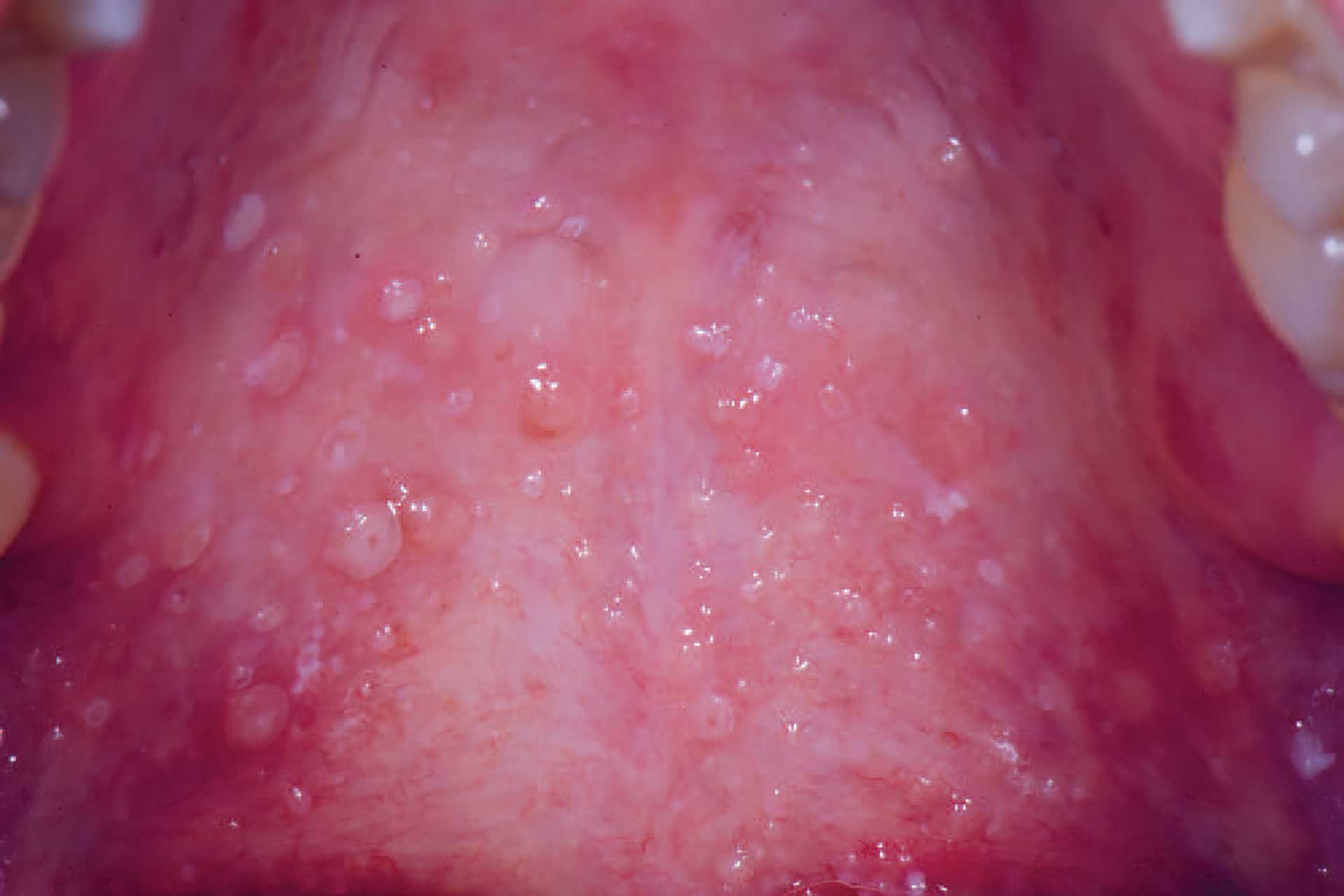
Figure 6. Mucoceles in the soft palate in a patient with cGVHD.
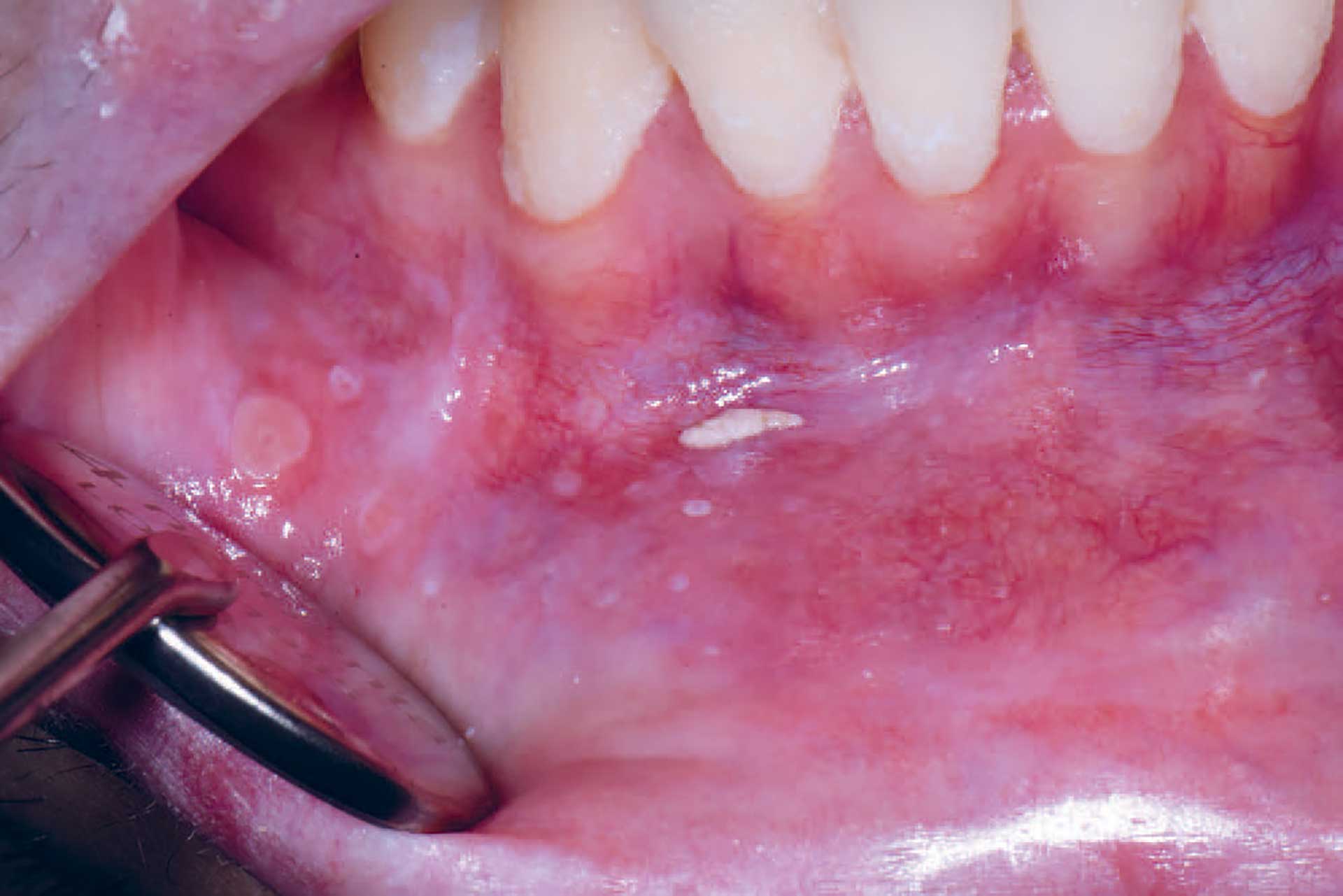
Figure 7. Mucoceles in a patient with cGVHD who also suffered from dry mouth.
Oral cGVHD manifests as oral lichen planus-like lesions, including white striations, erythema, and ulcerations [33] (figure 3-5). Xerostomia and hyposalivation are common in patients undergoing HSCT due to polypharmacy, radiotherapy, chemotherapy and cGVHD (figure 6-7). Salivary gland cGVHD lacks validated diagnostic criteria, but persistent xerostomia, hyposalivation and mucoceles may be indications of cGVHD and are regarded as distinctive, but not diagnostic, features of cGVHD [34][35][36]. Chronic GVHD is associated with impaired wound healing, tissue repair and fibrosis. Additional complications of cGVHD include perioral fibrosis leading to limited mouth opening, sensitive, vulnerable, and painful oral mucosa, increased caries activity and aggravation of periodontal disease [37][38][39][40][41].
Pathogenesis
The pathophysiology of cGVHD is not completely defined, and pathobiological pathways are probably different for the organs and tissue sites involved. Overall, immunocompetent donor T cells respond to genetically different human leukocyte antigens [29]. The acute inflammatory phase is triggered by conditioning-induced pathogen-associated molecules and host tissue damage-associated molecules followed by a chronic expansion of T cells. Macrophages and B cells might also play a role.
Changes in the oral mucosa and minor salivary glands in GVHD may histopathologically resemble those seen in oral lichen planus and Sjögren’s syndrome, respectively. The lichenoid lesions are characterized as a band-like lymphocytic aggregate that accumulates in the basal membrane region with exocytosis and apoptosis [42]. In the minor salivary gland tissue, periductal and acinar lymphocytic infiltration is considered specific for cGVHD [43]. The infiltrate usually comprises both plasma cells and T lymphocytes [43].
Diagnosis
The diagnosis is based on clinical recognition, particularly the presence of lichenoid lesions, with a history of allogeneic HSCT.
Treatment
Patients have systemic calcineurin inhibitors (CNIs) as GVHD prophylaxis. These agents are tapered, aiming for discontinuation postallogenic HSCT. Mild cGVHD is treated with topical corticosteroids or calcineurin inhibitors, whereas systemic corticosteroids are used for patients with moderate to severe cGVHD [44][45]. As such, first-line treatment often includes a combination of corticosteroids with or without CNIs [45][46]. As many as 50% of cGVHD patients become steroid refractory and demand second-line treatment within the first two years post-HCT [45][46]. Second-line therapies might involve extracorporeal photopheresis, B-cell depletion, antimetabolite immunosuppressants, chemotherapy, and kinase inhibitors [45][46][47].
The dentist plays an important role in the management of patients with oral cGVHD since diagnosis and management rely on clinical surveillance, which might be accompanied by a biopsy. Patients with severe disease should be treated by hospital oral disease units or specialists in oral medicine. Treatment should be inserted in collaboration with the medical team around the patient [48].
Prognosis, complications, and prevention
A special focus should be on hyposalivation and the risk of caries development [48]. Prolonged severe oral cGVHD is classified according to the WHO as a potential malignant disorder with an elevated risk for malignant transformation, which needs to be recognized by dental professionals [49].
Viral infections in immunocompromised patients
The most common viral infections affecting the oral cavity belong to the herpesviridae group: herpes simplex virus1 and 2 (HSV-1, HSV-2), human herpes viruses (HHV-3, HHV-4 and HHV-5), human papillomavirus (HPV), enteroviruses (coxsackie virus and enterovirus) and human immunodeficiency virus (HIV). Oral viral infections usually manifest as either solitary or multiple blisters (vesicles) and ulcerations. The onset of primary infection is typically abrupt, and general symptoms such as fever and malaise may be present. HSV-1 and -2, HPV and coxsackie virus are examples of viruses producing primary oral lesions, while oral manifestations of HIV occur due to immunosuppression.
Herpesviruses are a family of DNA viruses. HSV-1 and 2 produce lesions of oral, labial, nasal and/or genital mucosa. The epidemiology of HSV-1 infection is undergoing a transition, with less exposure in childhood and more exposure in adulthood [50]. In immunocompromised individuals, herpes infections can have more severe symptoms and more frequent recurrences.
Varicella zoster virus (VZV or HHV-3) causes the classic childhood disease chickenpox infection as a primary infection and establishes latency in nerve ganglia. Reactivation of the virus or recurrent infection is called herpes zoster or shingles and is characterized by painful eruptions in the skin and/or oral cavity. In the trigeminal nerve area, unilateral blisters or vesicles are characteristic signs and are found especially on the palate, gingivae, buccal mucosa, and lateral tongue. For HSV, herpes zoster can have more severe symptoms and more frequent recurrences in immunocompromised hosts.
Epstein‒Barr virus (EBV or HHV-4) infects B cells and is responsible for infectious mononucleosis but is also associated with oral hairy leukoplakia, Burkitt lymphoma and nasopharyngeal carcinoma in immunocompromised patients.
Cytomegalovirus virus (CMV or HHV-5) causes asymptomatic infection in childhood. In immunocompromised patients, it may produce oral ulcerations.
Oral lesions in HIV-infected patients have been observed since the beginning of the epidemic. Approximately 10% of HIV-infected patients have oral manifestations as a first sign of the infection. These manifestations are not specific to HIV but to immunodeficiency due to HIV infection. Oral manifestations are classified as fungal, bacterial (HIV-associated periodontal disease) and viral infections (EBV and hairy leukoplakia, VZV, HSV).
Conclusion
The oral cavity may exhibit manifestations of a wide range of systemic disorders or their treatments. The development of more advanced medical treatments is a specific challenge. Dentists need knowledge regarding variations in the drugs used and their side effects on the oral cavity. Furthermore, with an aging population in Nordic countries, both the prevalence of systemic disorders and the need for treatment increase. There is a lack of guidance for dental practitioners on the oral side effects of new advanced medical therapies, which are poorly characterized and reported. Therefore, oral manifestations may be underdiagnosed.
A close collaboration with the patients’ medical team is important. Careful history taking, detailed drug history and a meticulous clinical examination by dentists may facilitate the determination of the underlying etiology of oral manifestations and allow for earlier diagnosis and intervention in medical health care settings.
References
Colella A, Yu E, Sambrook P, Hughes T, Goss A. What is the Risk of Developing Osteonecrosis Following Dental Extractions for Patients on Denosumab for Osteoporosis? J Oral Maxillofac Surg. 2023; 81(2):232-237.
Ehrenstein V, Heide-Jørgensen U, Schiødt M, Akre O, Herlofson BB, Hansen S, et al. Osteonecrosis of the jaw among patients with cancer treated with denosumab or zoledronic acid: Results of a regulator-mandated cohort postauthorization safety study in Denmark, Norway, and Sweden. Cancer 2021; 127(21): 4050-8.
Pageau SC. Denosumab. MAbs. 2009; 1(3): 210-5.
Markham, A., Romosozumab: First Global Approval. Drugs, 2019; 79(4): 471-476.
Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J Oral Maxillofac Surg, 2022; 80(5): 920-943.
Hadji P, Coleman RE, Wilson C, Powles TJ, Clézardin P, Aapro M, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016; 27(3):379-90.
Gaudet C, Odet S, Meyer C, Chatelain B, Weber E, Parmentier AL, et al. Reporting Criteria for Clinical Trials on Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review and Recommendations. Cells. 2022; 11(24).
Ristow O, Rückschloß T, Schnug G, Moratin J, Bleymehl M, Zittel S, et al. Comparison of Different Antibiotic Regimes for Preventive Tooth Extractions in Patients with Antiresorptive Intake-A Retrospective Cohort Study. Antibiotics (Basel). 2023; 1;12(6):997.
Reguad, C. Sur la nécrose des os atteints par un processus cancéreux et traités par les radiations. Cpmtes remdus des seances de la Societe de Biologie et de ses filiales, 1922; 25: 427-429.
Chronopoulos A, Zarra T, Ehrenfeld M, Otto S. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int Dent J, 2018. 68(1):22-30.
He Y, Ma C, Hou J, Li X, Peng X, Wang H, et al. Chinese expert group consensus on diagnosis and clinical management of osteoradionecrosis of the mandible. Int J Oral Maxillofac Surg, 2020; 49(3):411-419.
Støre, G, M. Boysen. Mandibular osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin Otolaryngol Allied Sci. 2000; 25(5):378-84.
Nabil S, Samman N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: a systematic review. Int J Oral Maxillofac Surg. 2011;40:229-243
Aarup-Kristensen S, Hansen CR, Forner L, Brink C, Eriksen JG, Johansen J., et al. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: risk factors and dose-volume correlations. Acta Oncol. 2019; 58(10):1373-1377.
Brokstad Herlofson B, Schiodt M, Larsson Wexell C. Acute and chronic inflammation, osteomyelitis, medication-related osteonecrosis of the jaw and osteoradionecrosis., in Nordic Textbook in Oral and Maxillofacial Surgery, S.G. Tore Bjørnland T., Rasmusson L., and Nørholt SE. Eds. 2021, Munksgaard: Copenhagen.
Marx, R.E. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983; 41(5): 283-8.
Delanian, S. and J.L. Lefaix.The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004; 73(2):119-31.
Martos-Fe rnández M., et al. Pentoxifylline, tocopherol, and clodronate for the treatment of mandibular osteoradionecrosis: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018; 125(5):431-439.
EMA. Advanced therapy medicinal products: Overview. EMA. 2023 [cited 2011; Available from: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview.
Mian, M. Sreedharan S, Kumar T. Osteonecrosis of the jaws associated with protein kinase inhibitors: a systematic review. Oral Maxillofac Surg, 2021; 25(2):149-158.
Vallina C, Ramírez L, Torres J, Casañas E, Hernández G, López-Pintor RM. Osteonecrosis of the jaws produced by sunitinib: a systematic review. Med Oral Patol Oral Cir Bucal. 2019; 24(3):e326-e338.
Li CC, Malik SM, Blaeser BF, Dehni WJ, Kabani SP, Boyle N, et al. Head and neck pathology. 2012; Vol.6(2),290-295.
France K, Yogarajah S, Alcino Gueiros L Valdez R, Mays J, et al. World Workshop on Oral Medicine VII: Oral adverse effects to biologic agents in patients with inflammatory disorders. A scoping review. Oral Pathol Med. 2023;52:1–8.
Motamed-Sanaye A, Khazaee YF, Shokrgozar M, Alishahi M, Ahramiyanpour N, Amani M. JAK inhibitors in lichen planus: a review of pathogenesis and treatments. J Dermatolog Treat. 2022; 33(8):3098-3103.
Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017; 23(2): 211-34.
Pavletic SZ, Martin PJ, Schultz KR, Lee SJ. The Future of Chronic Graft-Versus-Host Disease: Introduction to the 2020 National Institutes of Health Consensus Development Project Reports. Transplantation and Cellular Therapy, Official Publication of the American Society for Transplantation and Cellular Therapy. 2021; 27(6):448-51.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U et al. International Multicenter Standardization of Acute Graft versus Host Disease Clinical Data Collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016; 22(1):4-10.
Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009; 373(9674):1550-61.
Fall-Dickson JM, Pavletic SZ, Mays JW, Schubert MM. Oral Complications of Chronic Graft-Versus-Host Disease. J Natl Cancer Inst Monogr. 2019; 2019(53).
Mays JW, Fassil H, Edwards DA, Pavletic SZ, Bassim CW. Oral chronic graft-versus host disease: current pathogenesis, therapy, and research. Oral Dis. 2013; 19(4):327-46.
Carlens S, Ringden O, Remberger M, Lonnqvist B, Hagglund H, Klaesson S, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant. 1998; 22(8):755-61.
Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft versus host disease according to National Institutes of Health consensus criteria. Blood. 2011; 117(11):3214-9.
Bassim CW, Fassil H, Mays JW, Edwards D, Baird K, Steinberg SM, et al. Oral disease profiles in chronic graft versus host disease. J Dent Res. 2015; 94(4):547-54.
Fall-Dickson JM, Mitchell SA, Marden S, Ramsay ES, Guadagnini JP, Wu T, et al. Oral symptom intensity, health-related quality of life, and correlative salivary cytokines in adult survivors of hematopoietic stem cell transplantation with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010; 16(7):948-56.
Legert K, Remberger M, Ringden O, Heimdahl A, Dahllof G. Salivary secretion in children after fractionated or single-dose TBI. Bone Marrow Transplant. 2012; 47(3):404-10.
Alborghetti MR, Correa ME, Adam RL, Metze K, Coracin FL, de Souza CA, et al. Late effects of chronic graft-vs.-host disease in minor salivary glands. J Oral Pathol Med. 2005; 34(8):486-93.
Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010; 18(8):1039-60.
Haverman TM, Raber-Durlacher JE, Rademacher WM, Vokurka S, Epstein JB, Huisman C, et al. Oral Complications in Hematopoietic Stem Cell Recipients: The Role of Inflammation. Mediators Inflamm. 2014; 2014:378281.
Castellarin P, Stevenson K, Biasotto M, Yuan A, Woo SB, Treister NS. Extensive dental caries in patients with oral oral chronic graft-versus-host disease. Biol Blood Marrow Transplant.2012; 18(10):1573-9.
Atsuta Y, Suzuki R, Yamashita T, Fukuda T, Miyamura K, Taniguchi S, et al.Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014; 25(2):435-41.
Treister NS, Cook EF, Jr., Antin J, Lee SJ, Soiffer R, Woo SB. Clinical evaluation of oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(1): 110-5.
Carrozzo M. Understanding the Pathobiology of Oral Lichen Planus. Current Oral Health Reports. 2014; 1(3):173-9.
Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006; 12(1):31-47.
SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease.v Hematology Am Soc Hematol Educ Program. 2008: 134-41.
Saidu NEB, Bonini C, Dickinson A, Grace M, Inngjerdingen M, Koehl U, et al. New Approaches for the Treatment of Chronic Graft-Versus-Host Disease: Current Status and Future Directions. Front Immunol. 2020; 11:578314.
Wolff D, Fatobene G, Rocha V, Kröger N, Flowers ME. Steroid-refractory chronic graft-versus-host disease: treatment options and patient management. Bone Marrow Transplant. 2021; 56(9):2079-87.
Zeiser R, Lee SJ. Three US Food and Drug Administration-approved therapies for chronic GVHD. 2022; 139(11):1642-5.
Tollemar V, Garming Legert K, Sugars RV. Perspectives on oral chronic graft-versus-host disease from immunobiology to morbid diagnoses. Front Immunol. 2023; 28;14:1151493.
Wolff D, Radojcic V, Lafyatis R, Cinar R, Rosenstein RK, Cowen EW, et al. National institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transplantation and cellular therapy. 2021; 27(10):817-35.
Ayoub HH, Chemaitelly H & Abu-Raddad LJ. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med 2019;17: 57.
Corresponding author: Karin Garming Legert, Department of Dental Medicine, Karolinska Institutet, PO BOX 4064, SE 14104 Huddinge, Sweden. E-mail: karin.garming.legert@ki.se
Accepted for publication 11.10.2023
The article is peer reviewed
Legert KG, Wexell CL, Pedersen AML, Gale G, Tollemar V, Ruokonen H, et al. Oral manifestations of systemic disorders – Part 2. Nor Tannlegeforen Tid. 2024; 134: 114-22.
Keywords; Oral; systemic; diseases; manifestations
