Victoria Dawson, Elisa Kristin Arnarsdóttir, Leona Malmberg, Homan Zandi og Merete Markvart
Optimize your treatment outcome
Lecturer, DDS, ph.d. Department of Endodontics, Faculty of Odontology, Malmö University, Malmö, Sweden.
DDS, MSc, Private Practice in Reykjavik, Iceland, Faculty of Odontology, University of Iceland, Reykjavik, Iceland.
Lecturer, senior consultant, DDS. Department of Endodontics, Faculty of Odontology, Malmö University, Malmö, Sweden.
Assistant professor, DDS, ph.d. Department of Endodontics, Institute of Clinical Dentistry, University of Oslo, Oslo, Norway.
Associate professor, DDS, ph.d. Research area Cariology and Endodontics, Section of Clinical Oral Microbiology, Department of Odontology, University of Copenhagen, Copenhagen, Denmark.
Headlines
High clinical success rate is expected when each step of the root canal treatment adheres to high quality standard of care.
An aseptic working field is maintained throughout the treatment using a tightly placed rubber dam preventing microbial contamination.
Access cavity with adequate size and shape enables a straight-line entry to the root canals.
Chemomechanical preparation using chemically-active irrigants removes microbial products and dissolve necrotic tissues.
A root-filling material with adequate length and size without any voids obturate the root canals, and the final restoration is placed in a timely manner.
A successful outcome of the endodontic treatment is strongly associated with well performed treatment procedures. An adequate access cavity preparation which is correctly positioned, of adequate size and with straight-line access to the canals, is a prerequisite for the subsequent endodontic treatment procedures to be properly performed. Under aseptic conditions, after gaining access to the root canals, the working length is determined by electronic apex locator combined with radiographs, preferably after coronal flaring. The root canals are then cleaned and shaped, in the vast majority of cases rotary or reciprocating Ni-Ti instruments can be used. This is performed in conjunction with the use of an irrigation solution, usually sodium hypochlorite with a low concentration. Once the chemomechanical instrumentation has been thoroughly performed, the next essential step is filling of the root canals. A root filling of good quality, that is, ending within 2 mm from the radiographic apex and without any voids, is of significant importance for the outcome while the materials and techniques appear less important. Finally, the tooth should be permanently restored as soon as possible after root filling, to prevent fracture and reinfection. Provided that the treatment procedures have been adequately performed, high success rates can be expected.
Endodontic treatment aims to prevent or eliminate root canal infection and apical periodontitis (AP). A successful treatment, from the clinician´s perspective, is usually defined as radiographic normal periapical conditions and the absence of clinical signs and symptoms, while retention of the root filled tooth, functionality and absence of pain are amongst the most important factors from the patient’s perspective.
High success rates (normal periapical conditions) have been reported for teeth without preoperative AP [1][2][3] whereas the rate is slightly lower for teeth with AP [1][4]. A successful outcome of the endodontic treatment is strongly associated with well-performed treatment procedures, from aseptic work to the final restoration. In such cases, most periapical diseases show signs of healing 1 year postoperatively; nonetheless, non-healed cases may be followed up to 4 years, allowing them enough time to heal [5].
In the following article, the endodontic treatment procedures including their significance for the outcome will be reviewed.
Aseptic work
Current endodontic treatment protocols are aimed at eliminating microorganisms and preventing the introduction of new microorganisms into the root canal system. Since endodontic pathogens are mainly oral commensals, isolating the tooth from the oral environment with a rubber dam is a prerequisite for safe and effective endodontic practice [6]. To minimize the risk of contamination, the isolated tooth and rubber dam also need to be disinfected [6]. Furthermore, all materials and instruments used during root canal treatment should be applied in a sterile, or effectively disinfected, condition. Gutta-percha points should be disinfected before use by immersion in, for example, chlorhexidine, alcohol, or sodium hypochlorite prior to obturation [7][8].
There is some evidence on contamination from the dentist to the root canal system, since skin commensals, such as Cutibacterium acnes and Staphylococcus aureus, have been identified in endodontic infections [9][10]. Bacterial numbers have also been found to be significantly increased on gloves, suggesting a risk of contamination [11][12]. Therefore, keeping good hand hygiene is of utmost importance. A no‐touch policy should also be adopted, meaning that efforts should be made to avoid touching the parts of instruments and materials which come in contact with the root canals, to further decrease the risk of contamination.
Every measure taken to establish and preserve asepsis during treatment is of value, as any means of reducing the microbial burden may cumulatively increase the chance of a successful treatment outcome.
Access cavity preparation and locating the canals
The objective of access cavity preparation is to completely unroof the pulp chamber, remove the coronal pulp tissue, and locate all of the canals with straight-line access, while saving as much tooth structure as possible; for proper cleaning, shaping, and obturation of the root canal system [13]. If the access cavity preparation is too conservative there is a risk of not locating all the canals [14]. Iatrogenic errors and errors in cleaning and shaping of the root canals [15] are other consequences if the access cavity preparation is not done properly, which may adversely affect the outcome of the endodontic treatment [3][16][17].
Prior to the access cavity preparation is initated caries and defective restorations should be removed, to prevent bacterial contamination during the treatment and to better visualize fractures, if present. Furthermore, the restorability of the tooth should be assessed.
The shape of the access is determined by the internal anatomy of the pulp chamber; its shape is a reflection of the external shape of the tooth at the level of the cemento enamel junction (CEJ). The outline of the chamber, its position and the distance from the occlusal surface to the coronal portion of the chamber (for the projected depth of access) should be assessed on preoperative periapical and bitewing radiographs. The angulation of the tooth should also be evaluated, so the bur can be angled in the right direction to prevent procedural errors such as perforations.
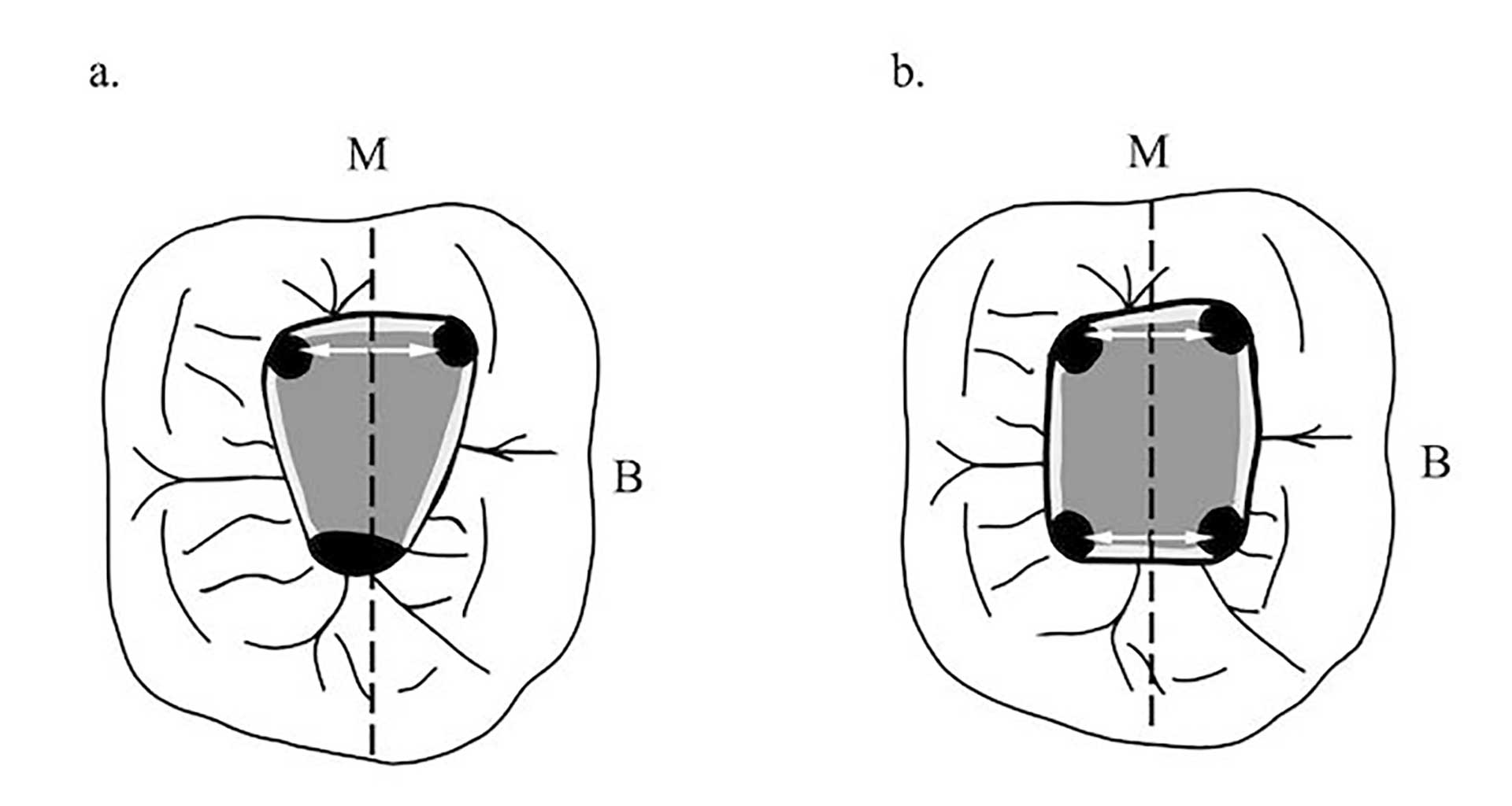
Figure 1. Excluding the maxillary molars, the canal orifices are equidistant from a line drawn through the pulp chamber floor in a mesial distal direction (dashed line) and lie on a line perpendicular to the mesial distal line (white arrows), except for maxillary molars. Mandibular molars (a, b) are shown as examples of this symmetry. The floor of the chamber is always darker than the walls.
Because many teeth in need of endodontic treatment have been heavily restored, using the occlusal anatomy as a reference point where to start accessing the tooth can lead to errors. As a result, it is preferable to follow the anatomy of the CEJ, which is the most reliable landmark for locating the pulp chamber, which is placed in the center of the tooth, at the level of CEJ [18].
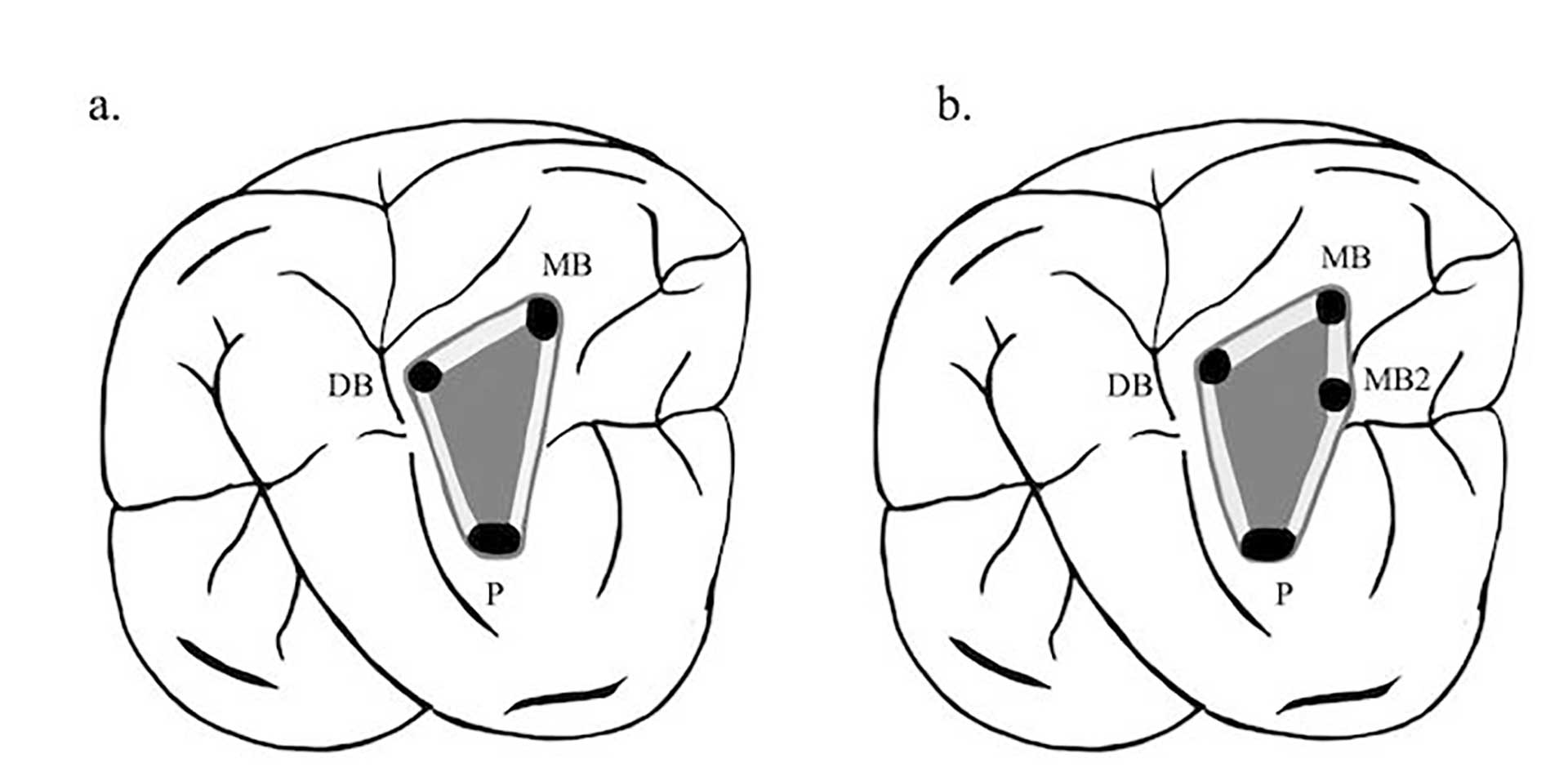
Figure 2. When accessing the maxillary molars an imaginary line can be drawn between the orifices of the tooth to form the molar triangle (a). The location of the MB2 usually skews the triangle, since its frequently located mesial to or directly on the line drawn between the MB and P orifices (b). Corresponding move of the access wall mesially needs then to be made.
The orifices are located at the junction of the walls and floor, at the angles in the floor-wall junction and at the terminus of the root developmental fusion lines. When locating the orifices, it is all about symmetry [18] (figure 1). When accessing the maxillary molars an imaginary line can be drawn between the orifices to form the molar triangle (figure 2a), however a second canal in the mesiobuccal root (MB2) is frequent [19], usually located mesial to or directly on the line drawn between the MB and P orifices (figure 2b).
The final shape of the access depends on the location of the orifices, and should only be refined after complete removal of the roof, when all of the pulp chamber floor is visualized and all of the canals have been found.
Root canal instrumentation and working length determination
After gaining access to the root canal system, the chemo-mechanical instrumentation can be initiated. The objective is to mechanically remove vital and/or necrotic tissue, shape the root canals in a way that the center of the root canal is maintained, and facilitate an optimal flow of irrigants so the entire surface of the root canal system is mechanically and or medically cleaned. In the vast majority of cases, rotary or reciprocating Ni-Ti file systems can be used. The flexibility of Ni-Ti instruments overcome several of the difficulties that may arise using only stainless steel instruments [20].
The instrumentation can be divided into four phases. Phase 1:Place a hand file into the root canal(s) and establish a glide path with hand files size 10-15. A glide path will improve the canal-centering ability of the later instruments. Phase 2: Coronal flaring with Ni-Ti rotary or reciprocating files securing straight line access. Straight-line access is, when a pre-bent hand file is able to reach the apical foramen or the first curve with no deflections and minimizes procedural errors during instrumentation. The motion is upwards going in contact with the root canal wall, usually towards the lingual shoulder in anterior teeth and away from the furcation in premolars and molars with only a minimal apical pressure. The level of pre-flaring may vary depending on curvatures and should stop before any curvature to avoid ledge formation. Phase 3: Confirm straight-line access with (hand) files and determine full working length (WL) using an electronic apex locator (EAL). The most accurate WL is obtained after coronal flaring [21]. Historically the WL has been determined using a combination of tactile sensation and radiographic images. However, using an EAL combined with radiographs ensures the most accurate WL, as the EAL takes into account local anatomical conditions such as the apical constriction, which we cannot see on radiographic images. Phase 4:Final apical preparation focusing on touching the entire periphery of the root canal. As the morphology and the dimension of root canals vary [22], it is crucial to choose the final file size based on the apical dimensions of the root canal. The apical size is determined with the first hand file that reaches WL passively and binds at the apex; from the first binding file, preparation is increased 2-3 sizes. E.g. if file size 20 binds, the canal is enlarged to size 35. The final file is handled in a crown-down approach.
Root canal irrigation and intracanal medication
The mechanical debridement has to be accompanied by an antibacterial irrigation solution to combat the microbial infection. The irrigation aims to remove and dissolve vital and necrotic tissue, microbes, dentin debris, smear layer, chemical disruption of biofilms, while causing no damage to the root canal wall or periradicular tissues [23][24]. Unfortunately, no single irrigant satisfies all the required characteristics. Countless irrigation solutions, such as saline, hydrogen peroxide, iodine, and chlorhexidine have been used; however, sodium hypochlorite (NaOCl) is considered the irrigant of choice [24]. NaOCl, in concentrations from 0.5 to 6%, has good antibacterial and tissue-dissolving properties [25]. Reducing its pH increases the bactericidal efficacy, while increasing its pH increases its tissue-dissolving ability [26]. While low and high concentrations have shown similar antibacterial effectiveness and clinical outcome, a lower concentration (0.5 to 1%) is less aggressive to periradicular tissues than higher ones [27]. High volume of irrigation, and long retention time of the irrigant has shown to positively influence root canal disinfection during preparation [28]. The volume and frequency of irrigation can compensate for the effect of concentration. However, there might exist a point of saturation, above which the increase in volume will no longer affect disinfection [29]. Ultrasonic activated irrigation (UAI) agitates the irrigation solution, activating their chemical actions, increasing the contact between irrigation fluid and root canal walls, ultimately leading to cavitation and disruption of biofilms within the root canal system [30]. Smaller side-vented irrigation needles (gauge 30) should be placed within 1 mm from WL to ensure fluid exchange with good turbulent flow [31]. During instrumentation a smear layer forms on the root canal walls and can be removed with chelator solutions such as ethylenediamine tetraacetic acid (EDTA) or citric acid [24]. Alternating between NaOCl and EDTA is not recommended as it reduces the antibacterial efficacy of the chlorine [23].
Intracanal medication with calcium hydroxide [Ca(OH2)] aims to supplement the antimicrobial effects of chemomechanical preparation, by eliminating microorganisms remaining in inaccessible areas for instrumentation and irrigation [32]. Noteworthy, within the limitations of studies, single- and multiple-visit endodontic treatment present similar post-operative pain, and healing rates [33]. In-between visits the access cavity is restored with a temporary filling material. To prevent bacterial leakage the thickness must be extended to a maximum [34].
Materials and techniques for root canal filling
Once the chemomechanical instrumentation is considered successful, filling of the prepared canal space is the subsequent essential step. The objectives are to prevent microbial products reaching the periapical area, to prevent inflow of tissue fluid to the root canal, and to entomb any remaining bacteria, by providing a tight seal along the entire canal space [6]. For that purpose, a core material, usually gutta-percha, in combination with a thin layer of sealer is used. Several root canal sealers are available, based on materials such as zinc oxide eugenol, epoxy resin and tricalcium silicate [35]. Techniques for root filling include, among others, single cone, lateral condensation and techniques where heat is applied to soften the gutta percha, for better adaption to the canal walls. Each technique has its advantages and disadvantages [36]. Materials and techniques for root filling have mainly been studied in a laboratory setting and clinical outcome studies on the topic are sparse. Thus, recommendation of any specific material or technique over another is not possible [37]. However, the technical quality of the root filling is of utmost importance. A good quality root filling, that is, ending within 2 mm from the radiographic apex and without any voids, significantly increases the chance for a successful outcome of the endodontic treatment [2][3][4]. Root fillings, ending >2 mm from the radiographic apex or overextended and/or with voids are associated with an increased risk of treatment failure [1][2][3]. Short root fillings and voids will allow survival and regrowth of possible remaining microorganisms in the unfilled spaces, and in case of coronal leakage reinfection is inevitable. Root fillings extended beyond the apex usually imply over instrumentation with a risk for extrusion of infected debris and difficulties in obtaining a tight seal in the apical part of the canal, increasing the risk of treatment failure [1][2][3].
For a successful outcome of the endodontic treatment, a root filling of good quality is of great significance while the materials and techniques seem to be less important. Still, the materials used should, ideally, be biocompatible, insoluble in tissue fluids, radiopaque, and removable in case of nonsurgical retreatment. It should have the ability to seal, but not shrink nor support bacterial growth [6].
Restoration of the root-filled tooth
Finally, a permanent restoration of high quality is of utmost importance for a successful outcome of the endodontic treatment [3][4]. The main objectives are to; provide an effective seal against microbial leakage and to improve the ability of the root filled tooth to withstand forces from biting and chewing - protecting it from fracture. Whether this is best achieved by direct or indirect restoration needs to be assessed in each individual case, with respect to a number of factors [38][39], (table 1). The type and material of the restoration should also involve preserving as much tooth structure as possible [38].
Clinical |
Patient-related |
|---|---|
|
Residual tooth structure amount quality (presence of cracks) Occlusal forces Position of the tooth Number of proximal contacts Endodontic and periodontal status of the tooth Strategic value of the tooth Status of adjacent teeth and the entire remaining dentition (caries, periodontal, restorative) Aesthetics |
Attitudes Expectations (e.g. aesthetics, longevity of the restoration) Financial status Preferences General health Dental health (e.g. caries risk) Social habits Motivation Compliance Other (e.g. dental fear) |
An effective seal against microbial leakage may be achieved by both direct and indirect restoration; no difference in success rates (normal periapical conditions) have been shown for root filled teeth restored with direct compared to indirect restoration [3][40][41]. However, further restorative treatment is more frequent in directly restored teeth than those indirectly restored [42], indicating a higher failure rate for direct restorations and potentially, a jeopardized seal against microbial leakage.
The survival rate is higher for root filled teeth restored with indirect compared to direct restoration [43][44][45][46]. A general recommendation for restoration of root filled teeth is not possible as the clinical evidence is insufficient [47], however, for root filled premolars and molars missing at least one proximal wall or in cases with a detectable crack, an indirect restoration may be considered [38]. Still, each case has to be individually assessed, taking clinical as well as patient-related factors into account.
Preferably, the tooth should be permanently restored as soon as possible after completion of the root canal treatment. Higher survival rates have been reported for teeth restored shortly after completion, in comparison to teeth restored with a delay of >60 days [48][49]. Root filled teeth without permanent restoration are at a higher risk for microbial leakage of the temporary filling [50] as well as for fractures [40]. It is not recommended to wait for healing of the periapical lesion before doing an indirect restoration, except for cases where the prognosis is uncertain.
Thus, a permanent restoration without delay, providing a good seal and protection of the root filled tooth is in favor of a successful outcome.
References
Sjogren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498-504.
Ricucci D, Russo J, Rutberg M, Burleson JA, Spångberg LS. A prospective cohort study of endodontic treatments of 1,369 root canals: results after 5 years. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:825-42.
Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. Int Endod J. 2011;44:583-609.
Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature -- Part 2. Influence of clinical factors. Int Endod J. 2008;41:6-31.
Ørstavik D. Time-course and risk analyses of the development and healing of chronic apical periodontitis in man. Int Endod J. 1996;29:150-5.
European Society of Endodontology. Quality guidelines for endodontic treatment: consensus report of the European Society of Endodontology. Int Endod J. 2006;39:921-30.
Gomes BP, Vianna ME, Matsumoto CU, Rossi Vde P, Zaia AA, Ferraz CC, et al. Disinfection of gutta-percha cones with chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:512-7.
Subha N, Prabhakar V, Koshy M, Abinaya K, Prabu M, Thangavelu L. Efficacy of peracetic acid in rapid disinfection of Resilon and gutta-percha cones compared with sodium hypochlorite, chlorhexidine, and povidone-iodine. J Endod. 2013;39:1261-4.
Sunde PT, Olsen I, Debelian GJ, Tronstad L. Microbiota of periapical lesions refractory to endodontic therapy. J Endod. 2002;28:304-10.
Niazi SA, Clarke D, Do T, Gilbert SC, Mannocci F, Beighton D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J Clin Microbiol. 2010;48:3859-69.
Niazi SA, Vincer L, Mannocci F. Glove Contamination during Endodontic Treatment Is One of the Sources of Nosocomial Endodontic Propionibacterium acnes Infections. J Endod. 2016;42:1202-11.
Zahran S. Mannocci F. Koller G. Assessing the Iatrogenic Contribution to Contamination During Root Canal Treatment. J Endod. 2022;48:479-86.
Gutmann J, Fan B. Tooth morphology, isolation and access. In: Hargreaves KM, Berman LH, Rotstein I, editors. Cohen´s Pathways of the Pulp. 11th ed. St Louis, MO: Elsevier;2016:142-4.
Shabbir J, Zehra T, Najmi N, Hasan A, Naz M, Piasecki L et al. Access Cavity Preparations: Classification and Literature Review of Traditional and Minimally Invasive Endodontic Access Cavity Designs. J Endod. 2021;47:1229-44.
Monsanto G, Smallwood ER, Gulabivala K. Effects of access cavity location and design on degree and distribution of instrumented root canal surface in maxillary anterior teeth. Int Endod J. 2001;34:176-83.
Weine FS, Kelly RF, Lio PJ. The effect of preparation procedures on original shape and on apical foramen shape. J Endod. 1975;1:255–62.
Crump MC, Natkin E. Relationship of broken root canal instruments to endodontic case prognosis: a clinical investigation. J Am Dent Assoc. 1970;80:1341–7.
Krasner P, Rankow HJ. Anatomy of the pulp chamber floor. J Endod. 2004;30:5-16.
Martins JNR, Marques D, Silva EJNL, Caramês J, Versiani MA. Prevalence Studies on Root Canal Anatomy Using Cone-beam Computed Tomographic Imaging: A Systematic Review. J Endod. 2019;45:372-86.
Glosson CR, Hailer RH, Dove SB, Rio CE. A Comparison of Root Canal Preparations Using Ni-Ti Hand, Ni-Ti Engine-Driven, and K-Flex Instruments. J Endod. 1995;21:146–51.
León-López M, Cabanillas-Balsera D, Areal-Quecuty V, Martín-González J, Jiménez-Sánchez MC, Saúco-Márquez JJ, et al. Influence of coronal preflaring on the accuracy of electronic working length determination: Systematic review and meta-analysis. J Clin Med. 2021;10:2760.
Markvart M, Darvann TA, Larsen P, Dalstra M, Kreiborg S, Bjørndal L. Micro-CT analyses of apical enlargement and molar root canal complexity. Int Endod J. 2012;45:273-81.
Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J. 2014;216:299-303.
Zehnder M. Root canal irrigants. J Endod. 2006;32:389-98.
Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35-40.
Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci 2006;11:147-57.
Verma N, Sangwan P, Tewari S, Duhan J. Effect of Different Concentrations of Sodium Hypochlorite on Outcome of Primary Root Canal Treatment: A Randomized Controlled Trial. J Endod. 2019;45:357-63.
Gazzaneo I, Vieira GCS, Pérez AR, Alves FRF, Gonçalves LS, Mdala I et al. Root canal disinfection by single- and multiple-instrument systems: effects of sodium hypochlorite volume, concentration, and retention time. J Endod. 2019;45:736-41.
Brito PR, Souza LC, Machado de Oliveira JC, Alves FR, De-Deus G, Lopes HP et al. Comparison of the effectiveness of three irrigation techniques in reducing intracanal Enterococcus faecalis populations: an in vitro study. J Endod. 2009;35:1422-7.
Mohammadi Z, Shalavi S, Giardino L, Palazzi F, Asgary S. Impact of Ultrasonic Activation on the Effectiveness of Sodium Hypochlorite: A Review. Iran Endod J. 2015;10:216-20.
Boutsioukis C, Lambrianidis T, Kastrinakis E, Bekiaroglou P. Measurement of pressure and flow rates during irrigation of a root canal ex vivo with three endodontic needles. Int Endod J. 2007;40:504-13.
Mohammadi Z, Dummer PMH. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697-730.
Su Y, Wang C, Ye L. Healing rate and post-obturation pain of single- versus multiple-visit endodontic treatment for infected root canals: a systematic review. J Endod. 2011;37:125-32.
Zandi H, Petronijevic N, Mdala I, Kristoffersen AK, Enersen M, Rôças IN et al. Outcome of Endodontic Retreatment Using 2 Root Canal Irrigants and Influence of Infection on Healing as Determined by a Molecular Method: A Randomized Clinical Trial. J Endod. 2019;45:1089-98.
Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M. Physical properties of 5 root canal sealers. J Endod. 2013;39:1281-6.
Trope M, Bunes A, Debelian G. Root filling materials and techniques: bioceramics a new hope? Endod Topics 2015;32:86-96.
SBU. Rotfyllning. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2010. SBU Utvärderar. [accessed Feb 28 2022]. Available from: https://www.sbu.se/sv/publikationer/SBU-utvarderar/rotfyllning/
Mannoci F, Bhuva B, Roig M, Zarow M, Bitter K. European Society of Endodontology position statement: The restoration of root filled teeth. Int Endod J. 2021;54:1974-81.
Dawson VS, Fransson H, Wolf E. Coronal restoration of the root-filled tooth – a qualitative analysis of the dentists´decision-making process. Int Endod J. 2021;54:490-500.
Chugal NM, Clive JM, Spangberg LS. Endodontic treatment outcome: effect of the permanent restoration. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:576-82.
Prati C, Pirani C, Zamparini F, Gatto MR, Gandolfi MG. A 20-year historical prospective cohort study of root canal treatments. A Multilevel analysis. Int Endod J. 2018;51:955-68.
Dawson VS, Isberg P-E, Kvist T, EndoReCo, Fransson H. Further treatments of root-filled teeth in the Swedish adult population: a comparison of teeth restored with direct and indirect coronal restorations. J Endod. 2017;43:1428-32.
Ng YL, Mann V, Gulabivala K. Tooth survival following non-surgical root canal treatment: a systematic review of the literature. Int Endod J. 2010;43:171-89.
Chen SC, Chueh LH, Hsiao CK, Wu HP, Chiang CP. First untoward events and reasons for tooth extraction after nonsurgical endodontic treatment in Taiwan. J Endod. 2008;34:671-4.
Landys Boren D, Jonasson P, Kvist T. Long-term survival of endodontically treated teeth at a public dental specialist clinic. J Endod. 2015;41:176-81.
Fransson H, Bjørndal L, Frisk F, Dawson VS, Landt K, Isberg PE, EndoReCo, Kvist T. Factors associated with extraction following root canal filling in adults. J Dent Res. 2021;100:608-14.
Sequeira-Byron P, Fedorowicz Z, Carter B, Nasser M, Alrowaili EF. Single crowns versus conventional fillings for the restoration of root-filled teeth. Cochrane Database Syst Rev. 2015 Sep 25;(9):CD009109. doi(9):CD009109.
Pratt I, Aminoshariae A, Montagnese TA, Williams KA, Khalighinejad N, Mickel A. Eight-Year Retrospective Study of the Critical Time Lapse between Root Canal Completion and Crown Placement: Its Influence on the Survival of Endodontically Treated Teeth. J Endod. 2016;42:1598-1603.
Yee K, Bhagavatula P, Stover S, Eichmiller F, Hashimoto L, MacDonald S, et al. Survival Rates of Teeth with Primary Endodontic Treatment after Core/Post and Crown Placement. J Endod. 2018;44:220-5.
Balto H. An assessment of microbial coronal leakage of temporary filling materials in endodontically treated teeth. J Endod. 2002;28:762-4.
Corresponding author: Victoria Dawson, email: Victoria.Dawson@mau.se
Accepted for publication 01.08.2022
The article is peer reviewed.
Dawson V, Arnarsdóttir EK, Malmberg L, Zandi H, Markvartd M. Optimize your treatment outcome. Nor Tannlegeforen Tid. 2023; 133: 108-12.
Keywords: endodontics, dental pulp diseases, periapical periodontitis, dental restoration, treatment outcome
