COVID-19 pandemic and oral health care: Cause-and-effect
Headlines
COVID-19 pandemic has caused concern about SARS CoV-2 transmission risk in dental care
SARS-CoV-2 is often found in the saliva of the infected subjects
Oral epithelial cells and salivary glands are susceptible to SARS-CoV-2
More stringent infection control practices have been adopted to control risk of transmission
Almost two years have now passed since the new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started to spread globally from Wuhan, China, leading to coronavirus disease (COVID-19) pandemic. COVID-19 symptoms vary from mild respiratory and gastrointestinal symptoms to severe pneumonia, and even death. New mutated variants have emerged throughout the pandemic and caused concern about the new clinical features they may possess, regarding transmissibility, severity of disease and vaccine effectiveness.
SARS-CoV-2 is transmitted mainly by respiratory secretions. It is frequently found in saliva of both asymptomatic and symptomatic infected patients. This has created tremendous concern about transmission during dental care among dentists and patients. Consequently, access to non-urgent dental care was highly restricted in early phases of the pandemic. This has caused an overall debt in access to dental care and risked timely dental treatment.
Oral epithelial cells and salivary glands are susceptible to SARS-CoV-2. Still, so far, there is no evidence of any SARS-CoV-2 oral disease manifestation confirmed by virological diagnostics. Poorer oral health, in particular periodontitis, has been suggested to aggravate COVID-19 possibly via increased aspiration of oral bacteria causing coinfections or due to periodontitis associated systemic inflammatory state. Further studies are needed to elucidate the possible effects of SARS-CoV-2 virus on oral and systemic health.
COVID-19 pandemic has set dental health care to face unforeseen challenges on one hand in terms of restrictions for providing dental care and on the other giving treatment safely without knowing the real risks for viral transmission. Oral epithelium and salivary glands are targets for SARS-CoV-2 and the virus is a frequent finding in saliva of both asymptomatic and symptomatic infected individuals. This has mounted considerable concern both among patients and dentists. The purpose of this review is to give an update on SARS-CoV-2 and to elucidate its possible effects on oral health as well as to give an overview on the effects of the COVID-19 pandemic on dental health care.
SARS-CoV-2 and COVID-19
In December 2019, an outbreak of disease caused by a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in Wuhan, China [1]. The disease it causes was termed Coronavirus disease-19 or COVID-19. SARS-CoV-2 turned out to have a significant potential for severe disease and to be far more successful in transmission from person-to-person compared to the other two recently emerged new coronaviruses, namely SARS-CoV-1 in 2002 and MERS-CoV in 2009 [2]. Already on March 11 2020, the World Health Organization (WHO) declared the outbreak a global pandemic.
Coronaviruses, belonging to the family Coronaviridae, are enveloped viruses with a large positive-sense single-stranded RNA genome of about 27-32 kilobases. The genome encodes four structural proteins named envelope glycoprotein spike (S), envelope (E), membrane (M) and nucleocapsid (N) protein and several non-structural proteins. [2]
SARS-CoV-2 is the ninth coronavirus known to infect humans [1]. All previous coronaviruses infecting humans are zoonotic, i.e. their origin is derived from animals [1]. The origins of SARS-CoV-2 is still to be unrevealed, but bats have been suggested to be the natural reservoir and the pangolin the possible intermediate host transmitting infection to humans. SARS-CoV-2 has also been proposed to be a manipulated virus escaped from a laboratory. During the pandemic SARS-CoV-2 has undergone many mutations that have increased adaptation in humans which would argue against it being intentionally manipulated to infect humans. These mutations include improved spike protein receptor binding. Currently the strongest body of evidence suggests that SARS-CoV-2 has its origin in animals and has been accidentally introduced to human. [3]
Many variants of SARS-CoV-2 have emerged during the pandemic. Mutations occur frequently in viruses, especially RNA viruses. Most of the mutations are irrelevant but some may greatly impact the natural properties of the virus. SARS-CoV-2 variants differ from each other clinically in transmissibility, clinical symptoms, immune escape properties, susceptibility to vaccines and performance of diagnostic tests [4] [5]. Based on these properties the variants have been classified as variants of concern (VOC), variants of interest (VOI) and variants under monitoring [6]. SARS-CoV-2 infections mounts antibodies against spike protein and nucleoprotein [5]. Of these, spike-specific antibodies neutralize the virus and provide protection against infection [5]. Therefore, current vaccines strategies mainly target the spike protein. Further, the mutations in spike protein have been shown to increase the infectivity and binding to cell surface receptor ACE2, the crucial initial step of infection [3]. The mutations in the spike protein region are of great interest and concern.
As of September 2020, the first VOC variants were identified. In addition to scientific nomenclature, VOC variants are designated by WHO with letters of the Greek alphabet. According to WHO, the VOCs currently include alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2) and omicron (B.1.1.529). To be defined as a VOCs, a lineage either shows increased transmissibility, changed epidemiology, increased virulence or change in clinical presentation or decreased effectiveness of public health and social measures or available diagnostics, vaccines and therapeutics. [6]
Pathogenesis
For the majority, COVID-19 has a relatively mild course in the form of fever, nasal discharge, sore throat, dry cough, headache, muscle ache, gastrointestinal symptoms and fatigue. Anosmia and ageusia may develop. More severe presentation of disease includes dyspnoea and pneumonia, and further sepsis syndrome and acute respiratory distress syndrome requiring intensive care, even death. [7]
In severe forms of infection, a marked dysregulation of the immune system, characterized by an excessive inflammatory response with cytokine storm, lymphopenia and neutrophilia, develops [8]. Inflammatory response leads to platelet and endothelial dysfunction which can result in thromboembolic complications [9].
SARS-CoV-2 binds to its host cell surface receptor angiotensin-converting enzyme 2 (ACE2) through spike protein S. ACE2 is widely expressed on epithelial cells, including upper and lower respiratory tract, oral cavity and gastrointestinal tract. The subsequent steps of viral replication require proteolytical activation by appropriate cellular proteases. S protein consists of subunits S1 and S2. S1 mediates the binding of the virus to the cell surface, whereas S2 anchors the virus and accounts for virus-cell membrane fusion. The entry and release of the nucleocapsid to the cytoplasm is dependent on the proteolytical activation by furin and the transmembrane protease serine 2 (TMPRSS) protease serine 2 (TMPRSS) on the plasma membrane and on cathepsin L on the endosomal compartment, respectively. Viral replication, transcription, translation and assembly of new viral particles take place in the cytoplasmic structures. Finally, mature viral particles are released from the plasma membrane. The expression of the spike glycoprotein at the host cell membrane may facilitate cell-to-cell fusion and subsequent formation of a syncytium permitting direct spread of SARS-CoV-2 between the cells. [7]
Diagnostics
The reference standard for SARS-CoV-2 testing is viral nucleic acid detection (RT-PCR) from nasopharyngeal (NP) swab samples. Recently, an increasing number of antigen detecting rapid diagnostic tests (AG-RDT) have become available [10] and these test are used in many countries as part of the testing strategy. The overall diagnostic accuracy of any test is greatly affected by the disease stage at which the sample is taken as well as the sample type. AG-RDTs vary in sensitivity. In average, the sensitivity is higher during the first week after symptom onset compared to the second week, which is compatible with high viral load typical for early phases of infection. [11]
Taking NP samples can be very unpleasant to the patients and requires specialized medical staff who are at risk of becoming infected when sampling. Alternatively, nostril or saliva samples could be used, but this has not yet been introduced to the routine practice. The advantage of saliva sample is that it can be taken non-invasively even by patients themselves. An agreement as high as 97.5% by RT-PCR analyses has been shown between NP and saliva samples [12] [13]. However, saliva testing demands a standardized collection protocol. A recent study showed that many ate or drank while in the line for testing and subsequently washed their mouths before saliva collection [14]. This resulted in impaired detection of SARS-CoV-2 as the viral load decreased due to mouth washing. The authors recommended avoiding eating, drinking, tooth brushing, and mouth washing at least 30 min before saliva sampling.
Origins of SARS-CoV-2 in the oral cavity
SARS-CoV-2 RNA and infectious virus can be detected from saliva in which up to 108 viral copies / ml has been detected [15]. The ACE2 receptor is expressed in the oral epithelial cells [16] [17] and the salivary glands [17] [18]. The ACE2 receptor expression level is particularly abundant in the epithelial cells of the tongue [16]. SARS-CoV-2 infection of oral epithelial cells and salivary glands has been confirmed by RNA and protein expression analysis [17]. Periodontal tissue was shown to be susceptible to SARS-CoV-2 in a post-mortem study in which five out of seven periodontal tissue autopsies were positive for SARS-CoV-2 RNA [19]. The longest time to sampling was 24 days from the onset of COVID-19 symptoms, suggesting that periodontal tissue may serve as a viral reservoir for lengthy periods of time. From saliva, viral RNA has also been detected for extended periods of time [15]. Moreover, SARS-CoV-2 RNA has been detected in gingival crevicular fluid with comparable degree of detection sensitivity with saliva [20].
Oral symptoms
Taste and olfactory disorders are suggested to result from inflammation or SARS-CoV-2 infection of neural or epithelial structures involved with these functions [21] [22]. Additionally, there has been many reports on possible oral mucosal manifestations of SARS-CoV-2 infection since the beginning of the pandemic [22] [23]. These include vesicobullous lesions, erosions, hemorrhagic and necrotic ulcerations, petechiae, swelling, bleeding, necrotizing gingivitis, and aphtous stomatitis. The most commonly sites affected are the tongue (38%), the labial mucosa (26%), and the palate (22%) [23]. Of particular interest is a case series published on isolated tongue ulcers (duration of ulcer 8,35 +/- 2,18 days) in 26 SARS-CoV-2 positive patients with mild respiratory symptoms [24) as high level of ACE2 receptor is expressed on the tongue. However, because the etiology of any of the reported oral lesions has not been confirmed by virological tests, it is unclear whether mucosal lesions actually represent an oral manifestation of SARS-CoV-2 infection or are complications of COVID-19 disease or its treatment. It seems currently more likely, that the oral mucosal lesions are associated with the immunosuppressive state induced by SARS-CoV-2 and the associated opportunistic coinfections (such as herpesvirus infections or candidiasis), COVID-19 associated vasculitis or hyperinflammatory response or represent erythema multiforme due to therapeutics used in patient care [21][23].
Furthermore, using global transcriptomic analysis ACE2 receptors and TMPRSS2 have been shown to be consistently expressed in the dental pulp both in normal and inflamed conditions [25]. It is currently unclear, whether COVID-19 has clinical impact on the diseases of the dental pulp.
COVID-19 and periodontitis
Periodontitis and COVID-19 are both characterized by a partially common pathophysiology with a background in a cytokine storm initiated by microbes [26]. Periodontitis as a comorbidity has been hypothetized to aggravate COVID-19 in vulnerable subjects with diseases such as diabetes, obesity and cardiovascular disease which are all known to associate with periodontitis [27]. Periodontitis increases the oxidative stress and inflammation in the body both locally and systemically, which may contribute to the increased morbidity and mortality of COVID-19. In a recent case-control study, moderate-to-severe periodontitis was found to associate with higher risk for intensive care unit admission, need for assisted ventilation and death of COVID-19 patients [28]. One explanation for this might be aspiration of periodontopathogenic bacteria which could result in enhanced inflammatory reaction and possibly to upregulation of ACE2 in the lower respiratory tract which both aggravate the course of infection [29].
Similar to other lung infections, COVID-19 pneumonia may get coinfected with other viruses, bacteria and fungi [30]. Here, oral cavity bacteria are of particular interest, as poor oral hygiene and periodontitis can complicate COVID-19 pneumonia [30]. The bacteria in the oral cavity can constitute a reservoir and can be transmitted to the lower respiratory tract by coughing and aspiration. Oral bacterial species such as Capnocytophaga and Veillonella have been detected in bronchoalveolar lavage fluid samples [31]. Pulmonary hypoxia, seen with COVID-19, promotes the growth of anaerobic and facultative anaerobic bacteria originating from the oral cavity, which could potentially aggravate the course of pneumonia. It is therefore vitally important that good oral hygiene is maintained to prevent these serious disease manifestations, especially in individuals who are at risk of complicated disease processes.
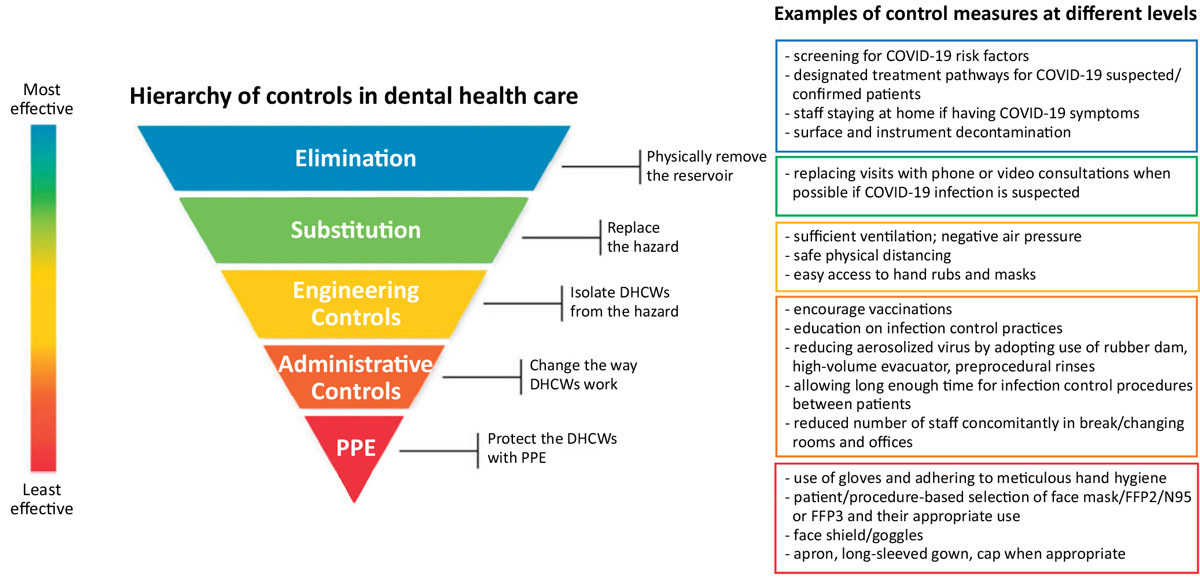
Figure 1. Decreasing occupational risks of SARS-CoV-2 transmission in dental clinic. Adapted from National Institute for Occupational Safety and Health (NIOSH) of the Centre for Disease Control and Prevention (CDC), USA and Volgenant et al, 2020 [35].
Preventing transmission of SARS-CoV-2 in the dental setting
SARS-CoV-2 is transmitted by inhalation or exposure of mucous membranes to infectious respiratory droplets or aerosols, or by contact between people, either directly or indirectly via surfaces. Thus, dental treatment is viewed as a high risk for SARS-CoV-2 transmission unless proper infection prevention and control measures are complied with [32] [33]. Therefore, also for professional reasons, SARS-CoV-2 vaccination is highly recommended for all dental health care personnel (DHCP) to provide protection both for the patients and DHCPs themselves.
The National Institute for Occupational Safety and Health (NIOH) is depicting occupational safety aspects in hierarchical levels. The idea is that the control measures at the top of the hierarchy are potentially more effective compared to those at the bottom (Figure 1.). During the pandemic, this strategy has been applied also to dental health care [34].
There is a general agreement on the main principles of preventing SARS-CoV-2 transmission in dental settings in most recommendations [32] [33] [35]. Before the appointment, all patients must be screened to rule out symptoms suggestive of COVID-19 and recent exposure to SARS-CoV-2. The same precautions also apply to all DHCPs. The possibility of postponing non-urgent treatment and routine dental care should be considered in areas with high community transmission. When entering the clinic both DHCPs and patients should wear a face mask and perform alcohol-based hand disinfection. Adequate safe distances among DHCPs and in between DHCPs and patients need to be maintained at all times when not wearing a face mask, for example during donning and doffing or eating. Number of staff in break and changing room as well as offices should be limited.
Personal protective equipment (PPE) always includes at least a surgical face mask, face shield/goggles, and gloves. Fluid resistant disposable gown and a cap are included in some of the guidelines. As an extra precaution, routine use of antiseptic preprocedural mouth rinse (usually 1% hydrogen peroxide or povidone-iodine) is included in some of the recommendations [32] [33], although evidence regarding its clinical effectiveness in preventing transmission of SARS-CoV-2 has not been shown. Continuous meticulous adherence to hand hygiene is important. After each patient thorough cleaning and surface disinfection of environmental surfaces and contact areas in the treatment area must be performed as well as sterilization or disinfection according to standard procedures of all dental instruments and equipment used during treatment. Further, it is important to ensure the proper function of high-volume evacuator to efficiently trap bioaerosols and sufficient room ventilation to dilute and remove the possible contaminates from the air.
COVID-19 suspected patients are treated according to designated conditions. Usually, the recommended PPE include an FFP2/N95 or FFP3 respirator, face shield, a fluid resistant disposable gown and a cap.
Aerosol generating procedures
Most attention has been paid to the risk of transmitting SARS-CoV-2 during aerosol generating procedures (AGP). WHO has defined AGP in oral health care as all clinical procedures that use spray-generating equipment such as three-way air/water syringe, ultrasonic scaler, and rotating instruments [32]. High-speed contra-angle hand piece (HSCAH) generates significantly less bioaerosols than the air turbine [36]. When Φ6-bacteriophage was used as a surrogate for SARS-CoV-2, it was further demonstrated that the use of a high-volume evacuator or a rubber dam removed almost all bioaerosol when HSCAH was used, while the reduction was not quite as efficient with air turbine [36]. The efficiency of evacuators is determined by the proximity of the evacuator to the working environment, suction strenght and number of evacuators [37], thus care should be paid to both meticulous use of the evacuator as well as its capacity. Most microbiological contamination from bio-aerosols and splashes is found within 1 m from the oral cavity [38]. Interestingly, the major source of the bioaerosol microbial load appears to originate from the dental irrigant instead of the oral microbiota. In a recent study, 78% of the microbial findings were compatible with dental irrigant water, while saliva derived microbes accounted for a median of 0% to aerosol microbiota and no SARS-CoV-2 nucleic acid was discovered though some of the patients had virus in their saliva [39]. More studies are needed to address the actual SARS-CoV-2 transmission risks associated with dental bioaerosols and the means to minimize them. The above findings suggest that the bioaerosols display a lesser transmission risk than previously believed and that the risk can further be decreased by rather simple means.
Some guidelines recommend avoiding AGPs whenever possible while most recommendations allow them in non-COVID-19 patients. When dental procedures are performed, high evacuation suction should always be used and in addition, a rubber dam whenever applicable. Four-handed dentistry is recommended. Using a pre-procedural antiseptic mouth rinse before AGP is included in some guidelines [33]. Using adequate personal protective equipment is mandatory. There is, however, disagreement on whether surgical masks, FFP2/N95 or FFP3 respirators should be used during AGP for non-COVID-19 patients while FFP2/N95 or FFP3 respirators are recommended for all suspected/confirmed COVID-19-patients [32] [33] [40]. Irrespective of type, correct donning and removing personal protective equipment is important to avoid microbial contamination [33] [35].
The background for the variation in specific recommendations in guidelines may be due to different risk assessments and the lack of scientific evidence on the effect of individual measures of infection prevention and control. In a recent review and subsequent consensus assessment regarding the guidelines on infection control and prevention during COVID-19 pandemic, data from 30 European countries were retrieved and analyzed [41] [42]. There was general agreement among recommendations for triage, mouth rinse, and PPE during AGP when treating potential COVID-19 patients. In contrast, recommendations on PPE for non-AGP treatment varied considerably. This reflects the limited availability of scientific evidence regarding transmission risk during non-AGP procedures.
Very encouraging studies from the initially seriously affected regions i.e. Wuhan in China and Lombardy in Italy have shown, that oral health care workers were at low risk of acquiring COVID-19 when adhering to standard infection control procedures combined with additional precautions as summarized above. Similar results were obtained from a 6-month prospective study in the United States. Thus, this far, these infection prevention and control measures have proved adequate for controlling the occupational risk of COVID-19 in dentistry even in areas with a high level of community transmission [43] [44].
The consequences of the pandemic for oral health and oral health care professionals
Dentistry has been viewed as a risk occupation and having dental care as a risk contact for patients for contracting SARS-CoV-2. Therefore, the total closure of dental practices for certain periods of time was recommended in several countries during the pandemic as well as postponing non-urgent dental care [32] [46]. The changed patients´ behavior has in part affected seeking for dental care. Some patients do not want to cause burden to the health care system, and others are afraid of contracting the virus during the visit. Poorer economical situation can affect both the patients and society´s resources for dental care. Dental personnel has been advocated new tasks, such as taking coronavirus samples, and also this has decreased resources available. The pandemic has decreased dentists´ income due to restrictions and possible quarantines after incidental exposure to SARS-CoV-2 either at or outside of work.
All in all, the pandemic has inevitably led to delays in access to non-urgent dental care. Delays of inspection and care may worsen chronic oral diseases, such as periodontitis. Poor oral hygiene and periodontitis may have led to aggravation of COVID-19 in some of the patients. Difficulties in accessing dental clinics may have increased the use of analgesics and antibiotics as a substitute for dental treatment. Postponing non-urgent care has been reported to delay oral cancer diagnosis [47], which may have dramatic consequences regarding the need for more morbid treatment and even patient survival.
Conclusions
Currently there are no indications to suggest that SARS-CoV-2 infection would compromise oral health directly. Instead, poor oral hygiene and increased microbial burden in untreated periodontitis may aggravate COVID-19 by enhancing the inflammatory reaction in the body and contributing to lung coinfection by aspiration. Thus, restricted access and decreased seeking for dental treatment constitute a risk for oral and systemic health. The pandemic has profoundly affected dental profession and dentists´ view about work-related infection risks management. The availability of SARS-CoV-2 vaccines together with appropriate infection prevention and control measures enable safe dental treatment for both patients and dental personnel.
References
Li J, Lai S, Gao GF, Shi W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature. 2021; 600(7889): 408-18. doi: 10.1038/s41586-021-04188-6.
Cui J, Fang L, Shi Zheng-Li. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019; 17(3): 181-92.
Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO et al. The origins of SARS-CoV-2: A critical review. Cell. 2021; 184(19): 4848-56. doi: 10.1016/j.cell.2021.08.017.
SARS-CoV-2 variants of concern as of 16 December 2021: https://www.ecdc.europa.eu/en/covid-19/variants-concern
Jalkanen P, Kolehmainen P, Häkkinen HK, Huttunen M, Tähtinen PA, Lundberg R et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021; 12(1): 3991. doi: 10.1038/s41467-021-24285-4.
Tracking SARS-CoV-2 variants: www.who.int/en/activities/tracking-SARS-CoV-2-variants/
Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020; 41(12): 1100–15. doi: 10.1016/j.it.2020.10.004
Wong LR, Perlman S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses - are we our own worst enemy? Nat Rev Immunol. 2021; 26: 1-10. doi: 10.1038/s41577-021-00656-2.
Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021; 18(3): 194-209. doi: 10.1038/s41569-020-00469-1.
Scheiblauer H, Filomena A, Nitsche A, Puyskens A, Corman VM, Drosten C et al. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021. Euro Surveill. 2021; 26(44): 2100441. doi: 10.2807/1560-7917.ES.2021.26.44.2100441.
Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021; 3(3): CD013705. doi: 10.1002/14651858.CD013705.pub2.
Azzi L, Maurino V, Baj A, Dani M, d’Aiuto A, Fasano M et al. Diagnostic Salivary Tests for SARS-CoV-2. J Dent Res. 2021; 100(2): 115-123. doi: 10.1177/0022034520969670.
Pasomsub E, Watcharananan SP, Boonyawat K, Janchompoo P, Wongtabtim G, Suksuwan W et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. 2021; 27(2): 285.e1-285.e4. doi: 10.1016/j.cmi.2020.05.001.
Melo Costa M, Benoit N, Tissot-Dupont H, Million M, Pradines B, Granjeaud S et al. Mouth Washing Impaired SARS-CoV-2 Detection in Saliva. Diagnostics (Basel) 2021; 11(8): 1509. doi: 10.3390/diagnostics11081509
To KK, Tsang OT, Yip CC, Chan KH, Wu TC, Chan JM et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis. 2020; 71(15): 841-3. doi: 10.1093/cid/ciaa149.
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020; 12: 8. doi: 10.1038/s41368-020-0074-x
Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021; 27(5): 892–903. doi: 10.1038/s41591-021-01296-8
Song J, Li Y, Huang X, Chen Z, Li Y, Liu C et al. Systemic analysis of ACE2 and TMPRSS2 expression in salivary glands reveals underlying transmission mechanism caused by SARS-CoV-2. J Med Virol. 2020; 92: 2556-66. doi: 10.1002/jmv.26045.
Matuck FB, Dolhnikoff M, Maia GVA, Sendyk DI, Zarpellon A, Costa Gomes S et al. Periodontal tissues are targets for SARS-CoV-2: a post-mortem study. J Oral Microbiol. 2021; 13: 1848135. doi: 10.1080/20002297.2020.1848135.
Gupta S, Mohindra R, Chauhan PK, Singla V, Goyal K, Sahni V, et al. SARS-CoV-2 detection in gingival crevicular fluid. J Dent Res. 2021; 100(2): 187-93. doi: 10.1177/0022034520970536.
Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA et al. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021; 20(9): 753-61. doi: 10.1016/S1474-4422(21)00182-4.
Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N et al. Oral manifestations in patients with COVID-19: A living systematic review. J Dent Res. 2021; 100(2): 141-54. doi: 10.1177/0022034520957289.
Iranmanesh B, Khalili M, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID-19: A review article. Dermatol Ther. 2021; 34(1): e14578. doi: 10.1111/dth.14578.
Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS-CoV-2 infection: A case series. Oral Dis. 2020; Sep 5. doi: 10.1111/odi.13635.
Galicia JC, Guzzi PH, Giorgi FM, Khan AA. Predicting the response of the dental pulp to SARS-CoV2 infection: a transcriptome-wide effect cross-analysis. Genes Immun. 2020; 21(5): 360-3. doi: 10.1038/s41435-020-00112-6.
Sahni V, Gupta S. COVID-19 & periodontitis: The cytokine connection. Med Hypotheses. 2020; 144: 109908. doi: 10.1016/j.mehy.2020.109908.
Coke CJ, Davison B, Fields N, Fletcher J, Rollings J, Roberson L et al. SARS-CoV-2 Infection and Oral Health: Therapeutic Opportunities and Challenges. J Clin Med. 2021; 10(1): 156. doi: 10.3390/jcm10010156.
Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, Hssain AA, Nicolau B, Sanz M, Tamimi F. Association between periodontitis and severity of COVID-19 infection: A case-control study. J Clin Periodontol. 2021; 48(4): 483-91. doi: 10.1111/jcpe.13435
Takahashi Y, Watanabe N, Kamio N, Kobayashi R, Iinuma T, Imai K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J Oral Sci. 2020; 63(1): 1-3. doi: 10.2334/josnusd.20-0388.
Bao L, Zhang C, Dong J, Zhao L, Li Y, Sun J. Oral microbiome and SARS-CoV-2: Beware of lung co-infection. Front Microbiol. 2020; 11: 1840. doi: 10.3389/fmicb.2020.01840.
Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin Infect Dis. 2020; 71(15): 713-720. doi: 10.1093/cid/ciaa203
World Health Organization. Considerations for the provision of essential oral health services in the context of COVID-19. Interim guidance. 3 August 2020. WHO 2020. https://www.who.int/publications/i/item/who-2019-nCoV-oral-health-2020.1
Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. Infection Control Guidance. Updated Sept. 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html#print
Volgenant CMC, Persoon IF, de Ruijter RAG, de Soet JJH. Infection control in dental health care during and after the SARS-CoV-2 outbreak. Oral Dis. 2021 Apr; 27 Suppl 3: 674-83. doi: 10.1111/odi.13408.
European Centre for Disease Prevention and Control. COVID-19 infection prevention and control measures for primary care including general practitioner practices, dental clinics and pharmacy settings: first update 19 October 2020. ECDC: Stockholm; 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-infection-prevention-primary-care-dental-clinics-pharmacy-october-2020.pdf
Vernon JJ, Black EVI, Dennis T, Devine DA, Fletcher L, Wood DJ et al. Dental Mitigation Strategies to Reduce Aerosolization of SARS-CoV-2. J Dent Res. 2021; 100(13): 1461-1467. doi: 10.1177/00220345211032885.
Samaranayake LP, Fakhruddin KS, Buranawat B, Panduwawala C. The efficacy of bio-aerosol reducing procedures used in dentistry: a systematic review. Acta Odont Scand. 2021; 79: 69-80.
Zemouri C, Volgenant CMC, Buijs MJ, Crielaard W, Rosema NAM, Brandt BW et al. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol. 2020; 12(1): 1762040. doi: 10.1080/20002297.2020.1762040.
Meethil AP, Saraswat S, Chaudhary PP, Dabdoub SM, Kumar PS. Sources of SARS-CoV-2 and Other Microorganisms in Dental Aerosols. J Dent Res. 2021; 100(8): 817-823. doi: 10.1177/00220345211015948.
Clarkson J, Ramsay C, Richards D, Robertson C, Aceves-Martins M on behalf of the CoDER Working Group. Aerosol generating procedures and their mitigation in international dental guidance documents – A rapid review. 24 July 2020. https://oralhealth.cochrane.org/sites/oralhealth.cochrane.org/files/public/uploads/rapid_review_of_agps_in_international_dental_guidance_documents.pdf
Becker K, Gurzawska-Comis K, Brunello G, Klinge B. Summary of European guidelines on infection control and prevention during COVID-19 pandemic. Clin Oral Implants Res. 2021 Oct; 32 Suppl 21: 353-81.
Gurzawska-Comis K, Becker K, Brunello G, Klinge B. COVID-19: Review of European recommendations and experts’ opinion on dental care. Summary and consensus statements of group 5. The 6th EAO Consensus Conference 2021. Clin Oral Implants Res. 2021 Oct; 32 Suppl 21: 382-8
Nardone M, Cordone A, Petti S. Occupational COVID-19 risk to dental staff working in a public dental unit in the outbreak epicenter. Oral Dis. 2020 Sep 3. DOI: 10.1111/odi.13632
Meng L, Ma B, Cheng Y, Bian Z. Epidemiological investigation of OHCWs with COVID-19. J Dent Res. 2020; 13: 1444-1452.
Araujo MWB, Estrich CG, Mikkelsen M, Morrissey R, Harrison B, Geisinger ML et al. COVID-19 among dentists in the United States. A 6-month longitudinal report of accumulative prevalence and incidence. JADA. 2021; 152: 425-2.
Daly J, Black EAM. The impact of COVID-19 on population oral health. Commun Dent Health. 2020; 37: 236-8.
Arduino PG, Conrotto D, Broccoletti R. The outbreak of Novel Coronavirus disease (COVID-19) caused a worrying delay in the diagnosis of oral cancer in north-west Italy: The Turin Metropolitan Area experience. Oral Dis. 2021 Apr 27 Suppl 3: 742-3 Apr 19: 10.1111/odi.13362. doi: 10.1111/odi.13362.
Corresponding author: Hanna Välimaa, University of Helsinki, Department of Virology, Haartmaninkatu 3, FIN-00290 Helsinki, Finland. Email: hannamari.valimaa@helsinki.fi
Accepted for publication
This paper has been peer reviewed.
Välimaa H, Larsen T, Klinge B, Fiehn N-E. COVID-19 pandemic and oral health care: Cause-and-effect. Nor Tannlegeforen Tid. 2022; 132: 140–6.
Keywords: SARS-CoV-2, COVID-19, saliva, oral mucosa, salivary glands, infection control
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Hanna Välimaa., Tove Larsen., Björn Klinge., Nils-Erik Fiehn.. COVID-19 pandemic and oral health care: Cause-and-effect. Nor Tannlegeforen Tid. 2022;133:140-6. doi:10.56373/2022-2-6
