New perspectives in the diagnosis and treatment of periodontitis
Headlines
Periodontitis is a well-orchestrated disease of the tooth supporting tissues, in which the pathogens, pathogen-associated molecular patterns, and host-response play significant roles
Molecular markers of infection and host-response, and related biological-pathways can be used to diagnose periodontitis and also can be considered as actionable therapeutic targets
Periodontitis is a chronic inflammatory disease of tooth-supporting tissues characterized by the breakdown of periodontal attachment and alveolar bone. The onset age and the progression rate of the disease vary depending on the presence of inherited and acquired risk factors and oral hygiene measures. The relatively slow-progressing forms of periodontitis are usually diagnosed in the third to fourth decades of life, while aggressive forms of the disease can be detected in young adults. Periodontitis proceeds in an asymptomatic pattern, meaning that initial stages of the disease are relatively underdiagnosed. Today, with the improvements in technology, it is possible to detect sub-clinical changes in the periodontal tissues by measuring the levels of non-invasively collected host- or bacteria-originated proteins, i.e. periodontal biomarkers. The present narrative review aims to present the current evidence of the clinical use of infection- and inflammation-related proteins as biomarkers. The molecular markers that can be targeted in periodontal disease treatment will also be discussed.
Molecular signatures of periodontitis: From diagnosis to disease modification
Periodontitis is the inflammatory disease of tooth-supporting tissues. It has a microbial etiology and a chronic degradative character. Periodontitis is accepted as a multifactorial disease, meaning that the severity and extent of observed periodontal tissue degradation can be modified by several systemic and local risk factors [1].
While the pathogenesis of periodontitis shows similarities among the population, the initiation, progression, and remission of the disease show individual and site-specific variations. In addition, progression of periodontitis demonstrates a non-linear chaotic dynamical process [2]. Indeed, the phrase “once a periodontitis, always a periodontitis” indicates that even after successful treatment, patient will be under the increased risk of recurrent periodontitis and requires lifelong maintenance therapy [3]. Routine dental check-ups are strongly advised to detect periodontitis at its very early stages and inhibit its progression before an irreversible tissue damage occurs. However, full-mouth periodontal screening of large populations might be costly and time taking. To overcome that obstacle, one approach is to categorize the population based on its periodontal risk status [4] [5]. With that, it can be possible to personalize the preventive care and treatment measures by 1) monitoring the individuals at high-risk groups more often to diagnose periodontitis at its very early stages, 2) to apply adjuvant therapies to periodontitis patients with weakened host-response, and 3) to inhibit the risk of relapse after periodontal treatment, especially in susceptible patients. Unfortunately, such aims are not always achievable, as national oral health care systems do not necessarily cover frequent dental check-ups or adjuvant therapy applications, which bring significant cost to the patient over time.
Technological improvements over the past three-four decades enabled us to understand the periodontitis pathogenesis more than before. Increased sensitivities in biomarker detection methods and decreased methodological costs allowed researchers to study various bacteria- or host-originated proteins as diagnostic markers of disease or actionable therapeutic targets for treatment (Figure 1).
With the aid of the rapid developments in nano- and microfluid technologies, various point-of-care/lab-on-a-chip tests and paper-based/flow-cytometry based platforms were developed to be used in dental clinics [6]. In this review, aim will be to answer the following questions; 1) What is the role of diagnostic markers of periodontitis? 2) How can such markers add information to clinical diagnosis? 3) How can products aiming at modulating inflammation be used as an adjunctive tool to mechanical infection control procedures in periodontal therapy?
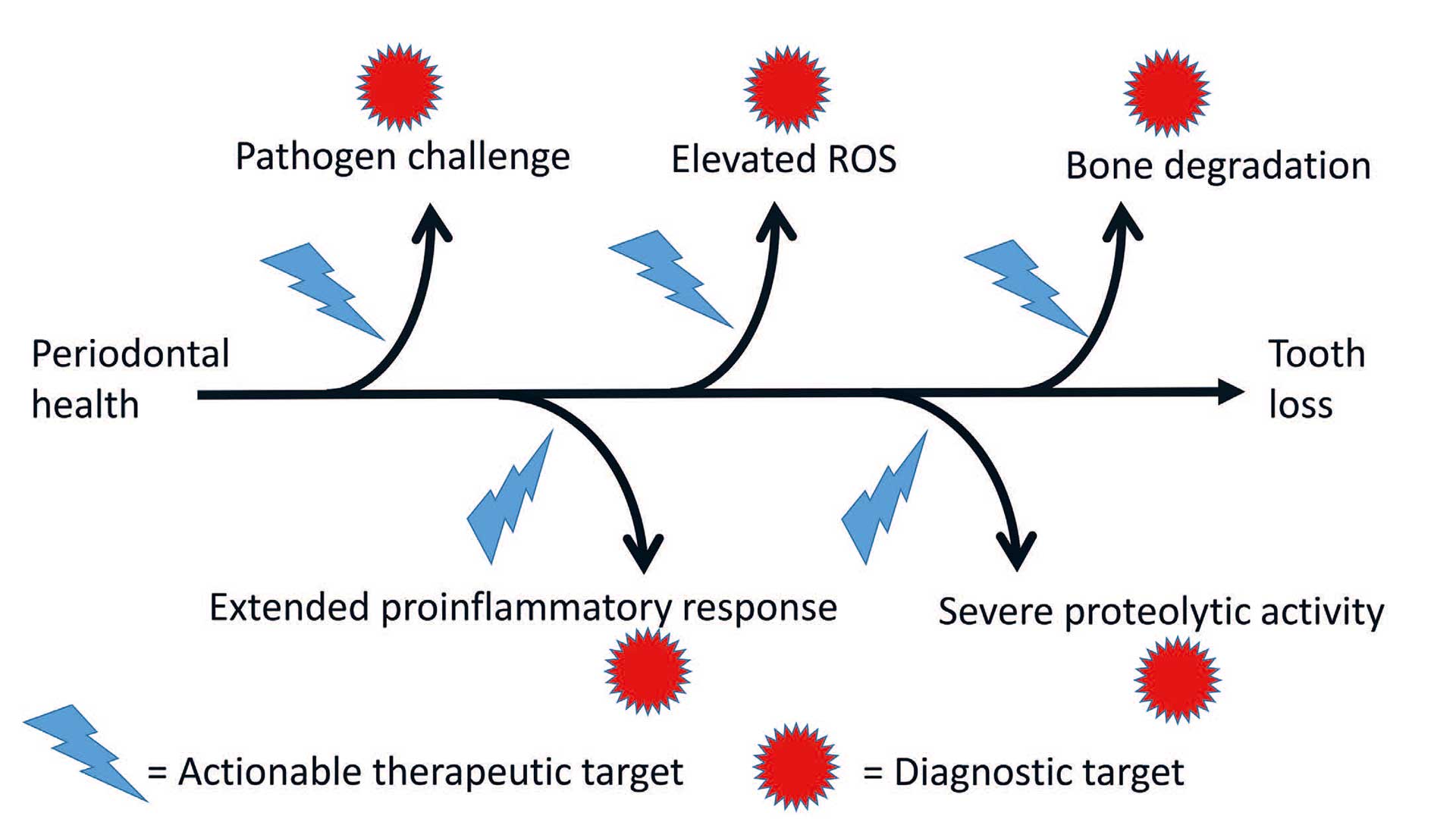
Figure 1. Important pathways in pathogenesis of periodontitis as diagnostic or therapeutic targets.
Diagnostic markers of periodontitis
Periodontitis is diagnosed and evaluated based on clinical and radiographic measurements, such as probing pocket depth (PPD), clinical attachment level (CAL) and bleeding on probing (BOP). It has been this way for more than 50 years and is still the case with the newest classification of periodontitis, where clinical and radiographic measurements continue to be the main determinants, used for staging and grading of periodontitis [7]. The main caveat of using PPD, CAL and radiographic bone loss alone is that they can only be used to identify periodontitis, when irreversible tissue damage has occurred. This is why diagnostic methods capable of identification of periodontitis at preclinical stages are urgently needed.
Periodontitis is a multifactorial disease and the subgingival microbiota and the host immune system are the main acts in the pathogenesis of periodontitis [8]. The first insight on the role of the oral microbiota in periodontal disease development was based on culture based microbial techniques. The development of culture independent molecular methods expanded the possibilities to grasp the complexity of the oral microbiota, which culminated with the microbial complex theory [9]. Since then, the continuous development of sophisticated molecular methods, based on next generations sequencing, has not only identified new periodontitis-associated bacteria, but also provided the possibility to fully reveal the complexity of the subgingival microbiota in periodontitis [10].
The keystone hypothesis introduced Porphyromonas gingivalis as the pathogen capable of orchestrating periodontitis disease progression, when being present even in low abundance subgingivally [11]. Thus, screening for P. gingivalis has been suggested as a molecular approach for detection of periodontitis. One example of this tactic was published in 2019, where Danish population-study reported that P. gingivalis was identified in 64% and 52% of saliva samples collected from patients with aggressive and chronic periodontitis, respectively, as compared to 8% of samples from periodontally healthy controls. Accordingly, identification of P. gingivalis in saliva was associated with relative risk (RR) of periodontitis in adults ranging from 6.5 to 8.1 [12].
While P. gingivalis seems to be the best bacterial marker candidate of adult periodontitis, another aspirant, Aggregatibacter actinomycetemcomitans, would probably be more suitable for screening of periodontitis in children and adolescents. In 2008, a two-year longitudinal study performed in adolescents from Morocco reported that subgingival colonization with A. actinomycetemcomitans at baseline was associated with a RR of 3.0 of having developed periodontitis two years later. Importantly, the RR increased to 18.1 if a specific subtype (JP2 clone) was the only variant of A. actinomycetemcomitans identified at baseline [13]. Indeed, the above-mentioned studies are proof of principles, in which the screening of subgingival and/or salivary bacterial abundance of specific bacterial species, such as P. gingivalis and A. actinomycetemcomitans, were suggested. However, in both studies there were patients with periodontitis, which were not colonized by P. gingivalis and A. actinomycetemcomitans, whereas some healthy controls were colonized by these pathogens. Therefore, routine screening of only one bacterial species at the dental office would result in a considerable amount of false-positive and false-negative identification.
The current explanatory model of periodontitis is based on the ecological plaque hypothesis, which implies that periodontitis develops as a consequence of dysbiotic interactions between the oral microbiota and the host immune system [8]. A good diagnostic application of ecological plaque hypothesis is the combinational use of bacterial and host-originated markers. Interestingly, such an approach was presented in 2011, where salivary levels of P. gingivalis, interleukin (IL)-1β and matrix metalloproteinase (MMP)-8 were used to calculate a cumulative risk score, which was proved to strongly associate with clinical periodontal status [4] [14]. Indeed, advanced molecular technologies such as metagenomics, metatranscriptomics, metaproteomics and metabolomics, collectively referred to as omics, are needed to fully portray the interaction between the microbiota and the host [10].
Microbial markers and clinical periodontal diagnosis: Current concepts
Until now, considerable amount of scientific evidence was gathered to conclude that polymicrobial biofilms of resident oral microorganisms in a “dysbiotic” relationship with the host, are instrumental in causation of periodontitis [15]. The use of state-of-art “omics” has enabled scientists to compile long lists of microorganisms present in biofilms or saliva of periodontitis patients and healthy individuals. These lists are not restricted to the “classical” periodontal pathogens that were known for decades (e.g. “red complex” species), but are extended to include species that were previously unknown or unsuspected to periodontitis. This knowledge has helped define the “core microbiome” of health or disease, which may consist of taxonomically different microorganisms that tend to share similar functional and metabolic properties [16]. It must be appreciated that “core microbiomes” can vary between individuals even in health [17], highlighting the importance of personalized dentistry.
The key question is whether any microbiological information can really be used to assist the clinical diagnosis of periodontitis within the dental office, which is a technically limiting point-of-care (PoC) environment for performing microbiological assays. Finding ways to accurately measure changes in the full or selective microbial composition and/or biosynthetic activity of dental plaque or saliva over sequential dental visits, may serve as an important diagnostic and prognostic parameter. If utilized efficiently, microbiological information will help to customize diagnosis and treatment planning, in line with the concept of personalized dentistry [18].
While the need for personalized healthcare is now more evident than ever, microbiological assaying in routine dental practice still poses significant practical challenges. At present there is no technically-friendly way to perform rapid full-scale screening of the oral microbiome at the dental PoC that can timely support the clinical decision. This may one day be feasible, as compact technologies for nucleic acid sequencing are constantly evolving. Nevertheless, qPCR assays and devices that can perform rapid molecular detection tests for a finite number of species at the dental PoC are currently underway [19]. These are tailored to detect well-known periodontal species as surrogate markers for monitoring the microbiome, and are competent in distinguishing between health and periodontal disease [20].
Host response markers and clinical periodontal diagnosis: Current concepts
After an intensive research over the past decades, salivary and GCF proteins of inflammation (IL-1β, macrophage inflammatory protein-1α), collagen degradation (MMP-8) and bone remodeling [osteoprotegerin (OPG), Receptor activator of nuclear factor kappa-Β ligand (RANKL) were identified as putative biomarkers of periodontitis [21]. The design of a quantitative chair-side diagnostics test that can be used in periodontitis diagnosis, however, is still a major challenge.
With the boost in GCF studies, various research groups attempted to differentiate active and inactive periodontal sites using GCF proteins as biomarkers. A successful example was the MMP-8 chairside test, which was developed to define the sites with periodontal degradation and also to follow the healing response after treatment [22] [23]. Even its predictive ability was demonstrated in independent studies, its production ended after some time.
One other commercialized tool was the IL-1 genotype based diagnostic test. This test was based on one pattern of IL-1 genetic polymorphisms, characterized by the IL-1A (+4845) and IL-1B (+3954) markers. Individuals with such polymorphism were found to be associated with periodontitis [24]. Even the IL-1-based genetic test was marketed heavily, its weak diagnostic utility and lack of literature support was criticized [25].
Among the chair-side/PoC -tests developed so far, the FDA (USA)- and EU –approved active MMP-8 PoC-lateral flow-immunotest discovered and developed by Sorsa et. al. [26] [27] exerts promising results in terms of diagnostic and prognostic characteristics regarding periodontitis and peri-implantitis [28] [29]. The aMMP-8 levels in mouth rinse and GCF/peri-implant sulcular fluid (PISF) indicate early collagenolytic inflammation around teeth and dental implants [26] [27] [28] [29]. Peri-implant sites showed a similar pattern of elevated aMMP-8 level in PISF to that observed in periodontitis sites, with a similar cellular source being mainly derived from inflammatory cells, particularly neutrophils [30]. Multi-national studies have shown that an active MMP-8 PoC test can detect initial periodontitis associated with single nucleotide polymorphisms of VDR and MMP-3 genes [26] [27] [28]. Although a chair-side/PoC aMMP-8 test could not discriminate between smokers and non-smokers with progressive periodontitis, it was demonstrated that this assay could predict the prognosis of smokers, in that elevated baseline-MMP-8 levels indicated a poor response to treatment, and sites that were non-responsive to treatment continued expressing high levels of aMMP-8 [30]. With its promising sensitivity and specificity values in diagnosis of periodontitis (at least two sites exhibiting PPD ≥5mm), aMMP-8 by lateral-flow chair-side/PoC immunoassay can be implemented into the new classification of periodontitis in the future. Yet, as periodontitis is a site-specific disease, chair-side/PoC immunoassays can only diagnose periodontitis at patient level and defining the sites with periodontal tissue degradation requires traditional diagnostic methods, i.e. PPD, CAL, and radiographic bone loss measurements.
Molecular markers of inflammation as actionable targets in periodontal therapy
In common sense, inflammation is accepted as the innate response against bacterial, chemical or physical trauma. Thus, by remodulating the inflammatory response, it might be possible to control the severity and extent of infection, modify the disease prognosis, shorten the treatment time, predict the treatment response, and increase the possibility of regeneration. During the last decades it was discovered that resolution of inflammation is not a passive, but an active and highly regulated process, and importantly, the molecules that mediate the programmed resolution of inflammation have been recovered [31]. A wide range of adjunctive interventions (including non-steroidal anti-inflammatories, statins, sub-antimicrobial dose doxycycline, resolvins and probiotics) have long been considered in managing inflammation as a part of non-surgical periodontal treatment. Many of those have been recently reviewed to evaluate whether there is sufficient available evidence for their efficacies. While studies show satisfactory clinical trends on some of these adjunctive therapies, further aspects need to be considered before full recommendation in routine clinical practice [32]. For instance, antimicrobial agents should be more thoroughly evaluated as for their potential to induce resistant bacterial strains, anti-inflammatories for their possible general side effects, and probiotics for consistency of the used formulations and overall clinical benefits. In principle, further multi-centered studies are warranted (Figure 2).
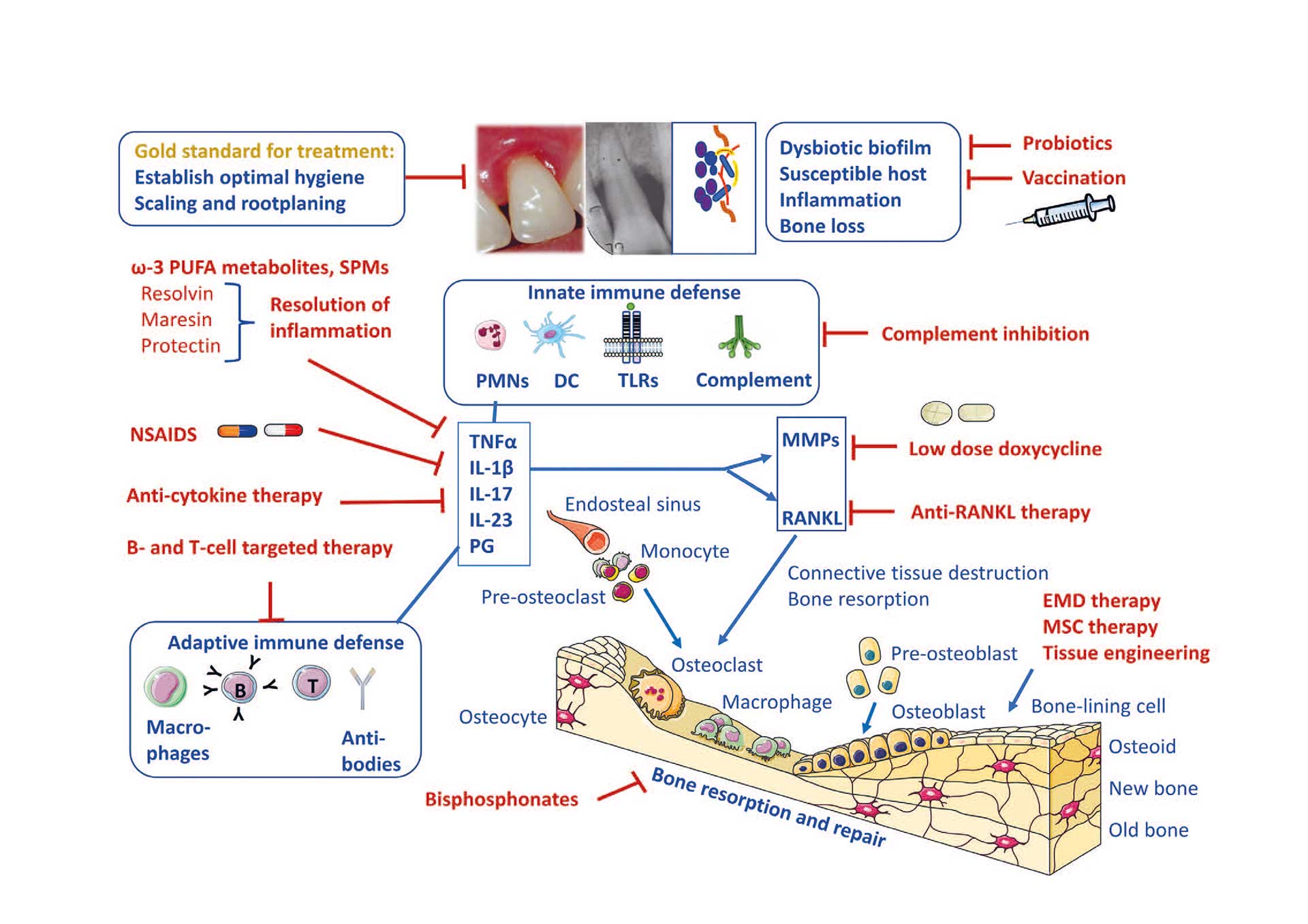
Figure 2. Conceivable opportunities for host modulation therapies of periodontal inflammation.
Stimulating resolution of inflammation
Periodontal inflammation is initiated by bacterial infection and is regulated by host chemical mediator proteins. These chemical mediator families, resolvins, protectins, maresins (ω-3 derived) and lipoxins (ω-6 derived), are actively generated from essential polyunsaturated fatty acids (PUFAs) and control the duration and magnitude of inflammation contributing to restore health [33]. Collectively, these molecules are termed specialized proresolving mediators (SPMs), and the families are expanding as new members are discovered [34]. Studies indicate that RvE1 directly acts on bone cells and promotes bone preservation. RvE1 has been shown to regulate inflammation and restore tissue homeostasis in periodontal disease in animal models [35].
Inhibiting the proteolytic activity (Low dose doxycycline)
MMPs possess collagenolytic properties and play a major role in periodontal tissue breakdown. It was recently demonstrated that the elevated salivary protease activities before periodontal treatment predict the steadiness of unresolved gingival inflammation [36]. Tetracyclines, in addition to their antibiotic properties, can modulate the activity of several MMPs through a number of non-antimicrobial mechanisms. Because MMPs also play a vital role in physiologic processes the intention is not to eliminate MMPs completely, hence a subantimicrobial dose of doxycycline has been launched as an adjunctive treatment for periodontitis. The efficacy of subantimicrobial dose doxycycline in routine clinical practice has yet to be determined [37].
Regeneration
Enamel matrix derivative (EMD) has remained one of the gold standards for tissue regeneration of lost periodontal tissue and bone defects. The major components of EMD are the amelogenin proteins, which are capable of supporting new periodontal ligament, cementum and alveolar bone formation [38]. EMD also affects the inflammatory and healing responses considerably. It substantially changes the OPG/RANKL balance in a positive direction by increasing OPG and decreasing RANKL. EMD also decreases IL-1β expression, increases prostaglandin E2 (PGE2) expression, proliferation of T-lymphocytes, bacterial and tissue debris clearance, and induce monocyte differentiation, fibroplasia and angiogenesis [38].
Mesenchymal stem cells (MSCs) have come into focus as potential candidates to rebuild lost periodontal tissue, often in combination with different types of scaffolds. MSCs have been considered promising because of their unique properties including stemness, proliferation, migration, multilineage differentiation and immunomodulation [39], showing anti-inflammatory effects. Both dental and non-odontogenic stem cells can potentially be applied, and the usefulness of stem cells from dental pulp, PDL and gingiva as well as bone MSCs and induced pluripotent stem cells are at present being evaluated for tissue engineering [40].
Anti-inflammatory therapies
Bisphosphonates are antiresorptive drugs that are used in prevention and treatment of osteoporosis. They bind to hydroxyapatite and interfere with the action of osteoclasts. Systemically administered bisphosphonates as an adjunct to scaling and root planing has been shown to inhibit alveolar bone loss and improve mineral density in humans with periodontitis, but an improvement of clinical inflammatory parameters has not been a consistently observed finding [37].
Nonsteroidal anti-inflammatory drugs (NSAIDS) have been considered for use in treating periodontitis not least due to their ability to block the production of prostaglandins. These drugs could improve the clinical outcome of mechanical periodontal treatment; however, they have serious unfortunate effects that prevent their use for periodontal therapy. Anti-cytokine therapy is used to treat inflammatory diseases including rheumatoid arthritis and has been proposed for periodontal treatment with IL-1 β and Tumor necrosis factor (TNF)-α as therapeutic targets. Nonetheless, use of anti-rheumatic drugs can have adverse effects on immunity. Furthermore, blockade of a single cytokine may not be effective if destructive inflammation is driven by a redundant cytokine network [37].
Conclusion
The information age (i.e. digital age) made significant changes in human life-style by making widespread use of technologies available. Today, a conversion from traditional methods to high-technology techniques in dental medicine is more close than ever. Clinicians need to update their knowledge and be ready to experience new age technologies in diagnosis and treatment of periodontitis to serve high-quality dental health services to their patients.
References
Könönen E, Gursoy M, Gursoy UK. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J Clin Med. 2019; 8(8): 1135.
Papantonopoulos G, Takahashi K, Bountis T, Loos BG. Mathematical modeling suggests that periodontitis behaves as a non-linear chaotic dynamical process. J Periodontol. 2013; 84(10): e29-39.
Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, Geisinger ML, Genco RJ, Glogauer M, Goldstein M, Griffin TJ, Holmstrup P, Johnson GK, Kapila Y, Lang NP, Meyle J, Murakami S, Plemons J, Romito GA, Shapira L, Tatakis DN, Teughels W, Trombelli L, Walter C, Wimmer G, Xenoudi P, Yoshie H. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018; 45 Suppl 20: S68-S77.
Gürsoy UK, Pussinen PJ, Salomaa V, Syrjäläinen S, Könönen E. Cumulative use of salivary markers with an adaptive design improves detection of periodontal disease over fixed biomarker thresholds. Acta Odontol Scand. 2018; 76(7): 493-496.
Petsos H, Arendt S, Eickholz P, Nickles K, Dannewitz B. Comparison of two different periodontal risk assessment methods with regard to their agreement: Periodontal risk assessment versus periodontal risk calculator. J Clin Periodontol. 2020; 47(8): 921-932.
He W, You M, Wan W, Xu F, Li F, Li A. Point-of-care periodontitis testing: Biomarkers, current technologies, and perspectives. Trends Biotechnol. 2018; 36(11): 1127-1144.
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol 2018; 89 Suppl 1: S159-S172.
Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, Ellwood R, Giacaman RA, Herrera D, Herzberg MC, Könönen E, Marsh PD, Meyle J, Mira A, Molina A, Mombelli A, Quirynen M, Reynolds EC, Shapira L, Zaura E. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol 2017; 44 Suppl 18: S5-S11.
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kenth Jr. RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998; 25(2): 134-144.
Feres M, Retamal-Valdes B, Gonçalves C, Cristina Figueiredo L, Teles F. Did Omics change periodontal therapy? Periodontol 2000 2021; 85(1): 182-209.
Hajishengallis G, Darveau RP, Curtis MA. The Keystone Pathogen Hypothesis. Nat Rev Microbiol. 2012; 10(10): 717–725.
Damgaard C, Danielsen AK, Enevold C. Porphyromonas gingivalis in saliva associates with chronic and aggressive periodontitis. J Oral Microbiol. 2019; 11(1): 1653123.
Haubek D, Ennibi OK, Poulsen K, et al. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008; 371(9608): 237-242.
Gursoy UK, Könönen E, Pussinen PJ, Tervahartiala T, Hyvärinen K, Suominen AL, Uitto VJ, Paju S, Sorsa T. Use of host- and bacteria-derived salivary markers in detection of periodontitis: a cumulative approach. Dis Markers. 2011; 30(6): 299-305.
Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E. The oral microbiome–an update for oral healthcare professionals. Br Dent J. 2016; 221: 657–666.
Zaura E, and Mira A. Editorial: the oral microbiome in an ecological perspective. Front Cell Infect Microbiol. 2015; 5: 39.
Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009; 9: 259.
Belibasakis GN, Bostanci N, Marsh PD, and Zaura E. Applications of the oral microbiome in personalized dentistry. Arch Oral Biol. 2019; 104: 7–12.
Mitsakakis K, Stumpf F, Strohmeier O, Klein V, Mark D, Von Stetten F, Peham JR, Herz C, Tawakoli PN, Wegehaupt F, Attin T, Bostanci N, Bao K, Belibasakis GN, Hays JP, Elshout G, Huisman RC, Klein S, Stubbs AP, Doms L, Wolf A, Rusu V, Goethel S, Binsl T, Michie A, Jancovicova J, Kolar V, Kostka M, Smutny J, Karpisek M, Estephan C, Cocaud C, Zengerle R. Chair/bedside diagnosis of oral and respiratory tract infections, and identification of antibiotic resistances for personalized monitoring and treatment. Stud Health Technol Inform. 2016; 224: 61–66.
Paqué PN, Herz C, Jenzer JS, Wiedemeier DB, Attin T, Bostanci N, Belibasakis GN, Bao K, Körner P, Fritz T, Prinz J, Schmidlin PR, Thurnheer T, Wegehaupt FJ, Mitsakakis K, Peham JR. Microbial analysis of saliva to identify oral diseases using a point-of-care compatible qPCR assay. J Clin Med. 2020; 9(9): 2945.
Kc S, Wang XZ, Gallagher JE. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. J Clin Periodontol. 2020; 47(3): 289-308.
Mäntylä P, Stenman M, Kinane D, Salo T, Suomalainen K, Tikanoja S, Sorsa T. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res. 2006; 41(6): 503-512.
Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006; 38(5): 306-321.
Kornman KS, Pankow J, Offenbacher S, Beck J, di Giovine F, Duff GW. Interleukin-1 genotypes and the association between periodontitis and cardiovascular disease. J Periodontal Res. 1999; 34(7): 353-357.
Huynh-Ba G, Lang NP, Tonetti MS, Salvi GE. The association of the composite IL-1 genotype with periodontitis progression and/or treatment outcomes: a systematic review. J Clin Periodontol. 2007; 34: 305–317.
Sorsa, T, Alassiri S, Grigoriadis A, Raisanen IT, Parnanen P, Nwhator SO, Sakellari D. Active MMP-8 (aMMP-8) as a grading and staging biomarker in the periodontitis classification. Diagnostics (Basel, Switzerland) 2020; 10(2): 61.
Sorsa T, Bacigalupo J, Könönen M, Pärnänen P, Räisänen IT. Host-modulation therapy and chair-side diagnostics in the treatment of peri-implantitis. Biosensors (Basel). 2020; 25; 10(5): 44.
Lähteenmäki H, Umeizudike KA, Heikkinen AM, Räisänen IT, Rathnayake N, Johannsen G, Tervahartiala T, Nwhator SO, Sorsa T. aMMP-8 point-of-care/chairside oral fluid technology as a rapid, non-invasive tool for periodontitis and peri-implantitis screening in a medical care setting. Diagnostics (Basel) 2020; 10(8): 562.
Golub LM, Räisänen IT, Sorsa T, Preshaw PM. An unexplored pharmacologic/diagnostic strategy for peri-implantitis: A protocol proposal Diagnostics (Basel). 2020; 10(12): 1050.
Gul SS, Abdulkareem AA, Sha AM, Rawlinson A. Diagnostic accuracy of oral fluids biomarker profile to determine the current and future status of periodontal and peri-implant diseases. Diagnostics (Basel). 2020; 10(10): 838.
Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007; 25: 101-137.
Donos N, Calciolari E, Brusselaers N, Goldoni M, Bostanci N, Belibasakis GN. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J Clin Periodontol. 2020; 47 Suppl 22: 199-238.
Mustafa M, Zarrough A, Bolstad AI, Lygre H, Mustafa K, Hasturk H, Serhan C, Kantarci A, Van Dyke TE. Resolvin D1 protects periodontal ligament. Am J Physiol Cell Physiol. 2013; 305(6): C673-679.
Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020; 64(3): 443-462.
Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007; 179(10): 7021-7029.
Gürsoy UK, Fteita D, Bikker FJ, Grande MA, Nazmi K, Gürsoy M, Könönen E, Belstrøm D. Elevated baseline salivary protease activity may predict the steadiness of gingival inflammation during periodontal healing: A 12-week follow-up study on adults. Pathogens. 2020; 9(9): 751.
Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol 2000. 2020; 84(1): 14-34.
Miron RJ, Sculean A, Cochran DL, Froum S, Zucchelli G, Nemcovsky C, Donos N, Lyngstadaas SP, Deschner J, Dard M, Stavropoulos A, Zhang Y, Trombelli L, Kasaj A, Shirakata Y, Cortellini P, Tonetti M, Rasperini G, Jepsen S, Bosshardt DD. Twenty years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol. 2016; 43(8): 668-683.
Wang M, Xie J, Wang C, Zhong D, Xie L, Fang H. Immunomodulatory properties of stem cells in periodontitis: Current status and future prospective. Stem Cells Int. 2020; 2020: 9836518.
Shanbhag S, Suliman S, Bolstad AI, Stavropoulos A, Mustafa K. Xeno-free spheroids of human gingiva-derived progenitor cells for bone tissue engineering. Front Bioeng Biotechnol. 2020; 8: 968.
Corresponding author: Ulvi Kahraman Gürsoy, Institute of Dentistry,
Lemminkaisenkatu 2, University of Turku, Turku, FI-20520 Turku, Finland.
Email: ulvi.gursoy@utu.fi
Accepted for publication 23.06.2021
This paper has been peer reviewed.
Gürsoy UK, Belibasakis G, Belstrøm D, Sorsa T, Bolstad A-I. New perspectives in the diagnosis and treatment of periodontitis. Nor Tannlegeforen Tid. 2022; 132: 122–8.
Keywords: Biomarkers; Infection; Inflammation; Periodontal diseases.
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Ulvi Kahraman Gürsoy., Georgios Belibasakis., Daniel Belstrøm., Timo Sorsa., Anne Isine Bolstad.. New perspectives in the diagnosis and treatment of periodontitis. Nor Tannlegeforen Tid. 2022;133:122-8. doi:10.56373/2022-2-4
