Association between oral infections and cardiovascular diseases
Headlines
Chronic oral infections are associated with cardiovascular diseases via direct and indirect mechanisms
Inflammation is an important link between oral infections and CVD
Oral infections and CVD share many common risk factors
Periodontal treatment has been proven to be beneficial for general health in addition to oral health
Abstract
The association between chronic oral infections and cardiovascular diseases (CVD) has been established in several extensive epidemiological studies. Most evidence is available on the association between periodontitis and atherosclerosis, and periodontitis has been recognised as an independent risk factor for CVD. The association between periodontitis and heart disease risk is independent of confounding factors such as patient’s smoking, age, sex, socioeconomic status or obesity. From the infected periodontal pockets of periodontitis patients, periodontal bacteria and their virulence factors may access the systemic circulation. In the arterial wall, periodontal pathogens have several proatherosclerotic effects. Periodontitis also causes low systemic inflammation which contributes to the development of atherosclerosis. In addition, periodontitis has an unfavourable effect on blood lipid levels lipid metabolism. There are also some genetic factors that may predispose to both periodontitis and CVD. Intervention studies have shown that with appropriate periodontal treatment, it is possible to impact CVD risk factors. Periodontal treatment has been shown to improve systemic levels of inflammatory (e.g. C-reactive protein and interleukins), thrombotic (fibrinogen) and metabolic (triglycerides, total cholesterol, HDL cholesterol, HbA1c, i.e. long-term blood glucose) markers and to improve blood vessel endothelial function. Periodontal treatment is thus beneficial for general health in addition to oral health.
Introduction
Chronic oral infections comprise caries, periodontitis, apical periodontitis, pericoronitis and mucous membrane infections. Most research evidence is available on the association between periodontitis and CVD. The role of apical periodontitis as a potential risk factor for heart disease has also been investigated in recent years. A separate article will be published on this subject.
In both periodontitis and atherosclerotic heart diseases, chronic inflammation and degradation of the extracellular matrix play a key role in disease development and progress. In atherosclerosis, lipids accumulate in the vessel wall, forming an atherosclerotic plaque. The chronic inflammation in the plaque contributes to plaque growth and rupture. Local inflammation may also damage the vessel endothelium, causing the formation of blood clots. As it grows in size, the atherosclerotic plaque strives to remodel itself aggressively in order to avoid significant narrowing of the vessel diameter and to ensure blood flow. However, the remodelling weakens the plaque and makes it prone to rupture. A local blood clot forms at the rupture site, which may cause the entire artery to be blocked. Atherosclerotic plaque rupture leads to a CVD event, such as myocardial infarction.
The association between oral infections and CVD has been established in several extensive longitudinal and cross-sectional studies. In the late 1980s, the first studies on this subject were published by Finnish groups (1,2). The study by Mattila et al. observed that oral health was clearly worse in patients with myocardial infarction than in control population even when subjects’ age, social class, smoking, blood lipids and diabetes were taken into consideration in the analyses (1). The study by Syrjänen et al. showed that periodontitis, periapical lesions and pericoronitis were more common in young and middle-aged stroke patients than in healthy controls (2).
Periodontitis has been recognised as an independent risk factor for CVD (3). However, in medicine, causality can only be established if strictly defined criteria are met (4). The notion of causality between periodontitis and cardiac diseases is supported by consistent research findings, convincing theoretical explanations and ample experimental evidence. However, establishing true causality between the two would require stronger scientific evidence, such as proof of the temporal sequence of events, i.e. the presence of periodontitis prior to heart disease.
The comprehensive oral infection burden has been illustrated with the help of various indices, some of which also take into account conditions such as caries, pericoronitis and retained dental roots in addition to marginal and apical periodontitis. Cross-sectional studies have shown these infection burden indices to be associated with coronary artery disease (5,6). In a 27-year follow-up study, the oral infection burden in childhood (caries and gingivitis) associated with the thickness of the carotid artery wall and the number of CVD risk factors in adulthood (7). There is also some indication of an association between diseases of the oral mucosa and CVD (8). For example, oral yeast infections may have potential systemic effects, as the treatment of prosthetic stomatitis has been shown to improve arterial function (9). As a whole, all oral infections may thus increase the risk of CVD through similar mechanisms as periodontitis.
Risk factors of chronic oral infections and CVD
Chronic oral infections and CVD share many common risk factors that modify predisposition to disease. Commonly recognised individual risk factors include age, male sex, smoking, heavy alcohol consumption, low socioeconomic status, diabetes, obesity, metabolic syndrome, nutrition-related factors and stress (10,11). In addition, similarities have been identified in the genetic profiles of periodontitis and CVD, and it is likely that in the future, common risk factors that are as yet unknown will be revealed. Tobacco is a significant risk factor for both periodontitis and CVD. It has multiple effects, including those on the circulation, microbiome, neutrophil function and cytokine production as well as on tissue regeneration potential (12).
Epidemiological studies have shown that periodontitis is a risk factor for many systemic diseases, such as diabetes, CVD and rheumatoid arthritis (13). The associations observed between periodontal diseases and CVD may be partly explained by the proinflammatory effect of obesity (13). Diabetes-related changes in, for example, neutrophil and macrophage function, cytokine secretion and wound healing also predispose to both periodontitis and CVD (14). These diseases may also partly be linked by certain genetic risk factors. For example, a certain variant of the VAMP8 gene is associated with both periodontitis and coronary artery disease (15). The gene in question affects cytokine secretion, glucose metabolism, blood coagulation and wound healing, among others. These are processes that are important for the disease mechanisms in periodontitis as well as CVD.
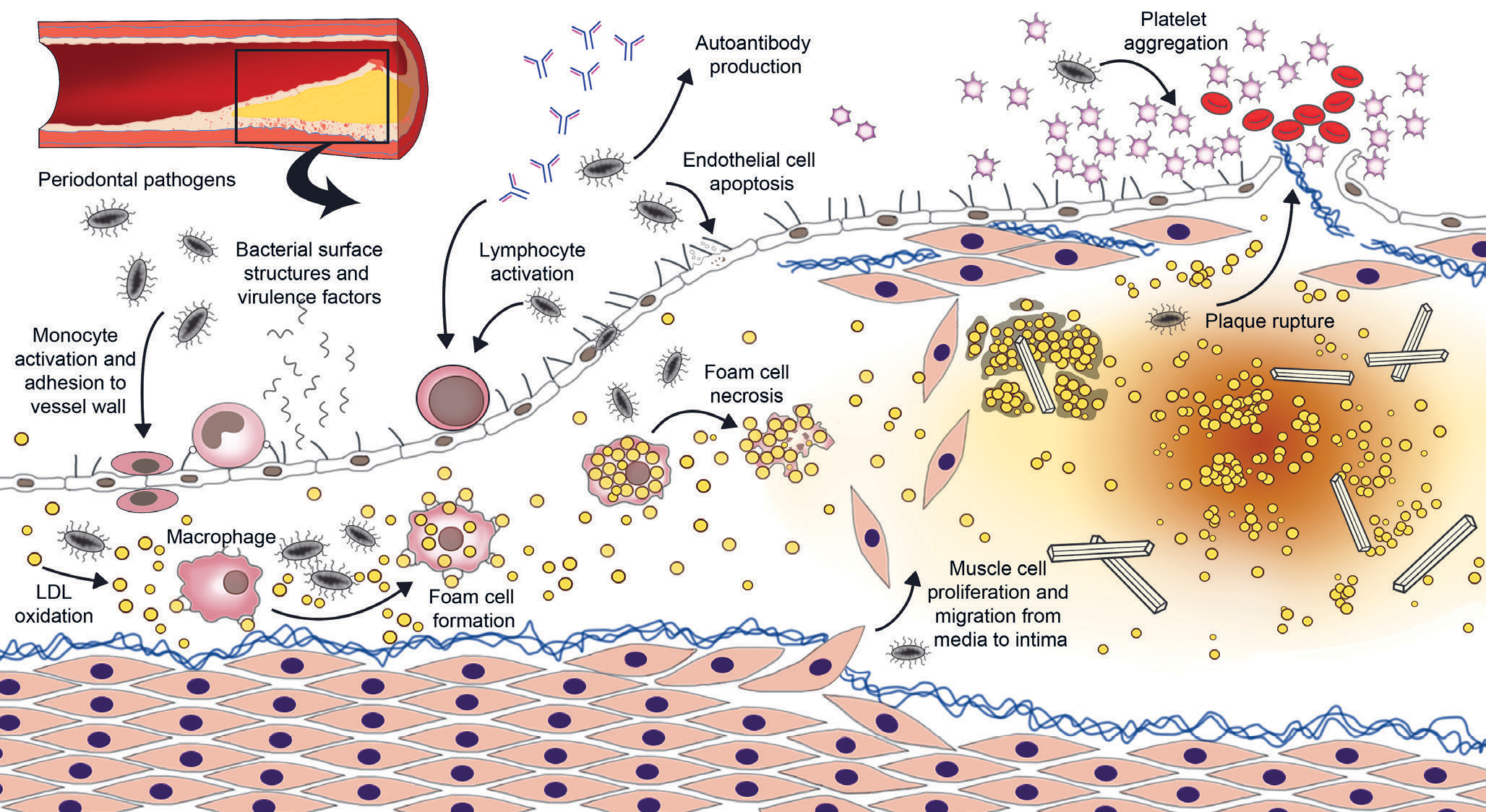
Figure 1. Schematic overview of the potential effect of periodontal bacteria on the development of an atherosclerotic plaque. Periodontal pathogens and their surface structures and virulence factors may enter the systemic circulation via inflamed periodontal pockets. In vessel walls, they contribute to atherogenesis via several mechanisms.
The mechanisms through which periodontitis is linked to CVD
The association of periodontitis with CVD has been explained by a number of mechanisms, which can roughly be classified into direct and indirect effects between the disease processes. The direct mechanisms include the effects of periodontal bacteria and their virulence factors on vessel walls while the indirect effects comprise the systemic inflammation and metabolic changes caused by periodontitis.
As periodontitis progresses, the proportion of pathogens in the oral microbiome increases. A diverse dysbiotic bacterial colony overactivates the local inflammatory response and attracts proteolytic enzymes to the site, which leads to periodontal tissue damage. In periodontitis, the production of proinflammatory cytokines, such as tumour necrosis factor (TNF) α, interleukin (IL) 1β and IL-6, is increased. As a systemic effect of these cytokines, the production of acute-phase proinflammatory mediators in the liver, such as C-reactive protein (CRP), increases further. (16)
Chronic infections like periodontitis increase low-grade systemic inflammation. This is known to promote the development of atherosclerosis, i.e. the formation of atherosclerotic plaques in the arterial walls, which increases the risk of blood clot formation, coronary artery disease and myocardial infarction (17,18). Changes in hormone levels may also have an impact on metabolic changes. For example, leptin levels have been observed to be elevated in both patients with periodontitis and those with coronary artery disease; this may interfere with immune response, lipid metabolism and bone regeneration (19).
Oral bacteria get into the bloodstream during daily activities, such as eating and brushing teeth. Some periodontal pathogens, such as Porphyromonas gingivalis, get around the immune system by invading endothelial cells, macrophages and dendritic cells. The bacteria reach the arterial walls as such from the bloodstream or hidden in leukocytes (16). Once attached to the arterial wall, the bacteria have several proatherosclerotic effects: they increase monocyte activation, adhesion molecule production by endothelial cells, LDL lipoprotein oxidation, foam-cell formation in vessel walls, smooth muscle cell proliferation, and endothelial cell apoptosis (20). Live periodontal pathogens have been detected in atherosclerotic plaques, and their involvement in the disease process is supported by several in vitro and animal studies (20). The effect of periodontal pathogens in the vessel wall is illustrated in Figure 1.
Pathogen virulence factors, such as proteases, adhesins and lectins also end up in the circulation. For example, gingipain, a protease secreted by P. gingivalis, is known to modulate the immune response and to increase platelet aggregation (16,20). The proinflammatory surface structure lipopolysaccharide (LPS, endotoxin) of gram-negative bacteria is a known risk factor for atherosclerosis that activates immunological response by binding to toll-like 4 receptor, for example. Oral infections may increase the level of endotoxemia, i.e. LPS in the blood, which increases the risk of heart disease (21). Periodontitis has also been observed to increase proatherosclerotic oxidative stress in the body, which leads to disturbed cell metabolism, autophagy and apoptosis (22).
Some of the pathogen structures, such as heat shock proteins (HSP) and the P. gingivalis protease gingipain, resemble the body’s own proteins. As a result, the antibody-mediated immune reaction targeting oral infections may cause the body to produce autoantibodies which increase the atherosclerotic inflammatory response and promote endothelial function impairment (23). Increased levels of antibodies against cardiolipin, phosphorylcholine and oxidised LDL have also been detected in patients with periodontitis (16,23). This impairs the coagulation system, increases systemic inflammation, and speeds up the formation of foam cells in arterial wall plaques (16,23).
Patients with periodontitis often have elevated blood LDL and triglyceride levels and reduced HDL lipoprotein levels (24). Excess LDL cholesterol accumulates in the vessel wall, speeding up atherosclerosis (Figure 1). In patients with periodontitis, the ability of HDL particles to remove cholesterol from the vessel wall is also impaired (24). Dyslipidaemia, i.e. impaired fat metabolism, is a known risk factor for atherosclerosis and one of the suggested mechanisms linking oral infections and heart disease (25). Oral infections have also been observed to activate the blood coagulation system, e.g. by increasing the production of fibrinogen and the aggregation tendency of platelets (20, 23).
In addition, experimental studies have shown that when swallowed with saliva, P. gingivalis has the ability to alter the gut microbiome and to modify the serum metabolite profile, increasing the risk of CVD, for example (26).
The mechanisms that explain the association between oral infections and atherosclerotic heart diseases are investigated globally and new connections between the two are constantly being discovered. Due to the inflammatory nature of both diseases it is possible that many of the metabolic disorders described above work in both ways. Based on current knowledge, inflammation is thought to be the most important connecting mechanism between oral infections and CVD.
The effect of oral infections and periodontal treatment on inflammatory markers
Besides dyslipidaemia, the low-grade inflammation commonly seen in patients with periodontitis is also associated with elevated leukocytes and serum glucose levels (27). Systemic levels of CRP, IL-6, TNF-α and fibrinogen are elevated in patients with periodontitis (27). The increase in high sensitivity CRP (hsCRP) seen in periodontitis patients is moderate and equivalent to the hsCRP level that predisposes to CVD: according to one study, the average hsCRP among patients with periodontitis was 2.6 mg/l, compared to 1.78 mg/l in healthy controls (28). Studies show that the relative risk of coronary artery disease, myocardial infarction and stroke is low when hsCRP is below 1.0 mg/l, moderate when hsCRP is 1.0¬–3.0 mg/l, and high when hsCRP exceeds 3.0 mg/l (29). The blood levels of inflammatory and metabolic markers have been observed to correlate with the number of periodontal pockets (27).
It is possible to impact CVD risk factors with appropriately delivered periodontal treatment. Anti-infective periodontal treatment includes the professional removal of biofilm and retentive factors, such calculus and restoration overhangs. The effects of periodontal treatment are illustrated in Figure 2. It has been shown to improve the plasma levels of inflammatory (hsCRP, IL-6, TNF-α), thrombotic (fibrinogen) and metabolic (triglycerides, total cholesterol, HDL cholesterol, HbA1c, i.e. long-term blood glucose) markers and to improve blood vessel endothelial function over a 6-month follow-up (30,31). It should be borne in mind that endothelial function becomes impaired already in the early stages of atherosclerosis (30). Periodontal treatment has also been observed to promote the vasodilatory capacity of arteries (32). It appears that particularly patients suffering from CVD and/or diabetes at the onset benefit from the treatment (31). In addition, a longitudinal study was able to show that periodontal healing slowed down thickening of the carotid artery wall (33). Patients with poor response to periodontal treatment had an increased risk of myocardial infarction, stroke or severe heart failure over a mean follow-up of 17 years (34). However, the findings of a Cochrane review show that the evidence of the preventive effect of periodontal treatment in the primary and secondary prevention of cardiac events is still weak, which is why further research is warranted (35).
Elevated blood glucose, metabolic syndrome and diabetes are significant risk factors for CVD. Meta-analyses show that conservative periodontal treatment lowers the level of glycosylated haemoglobin (HbA1c, so-called long-term blood glucose) both statistically and clinically significantly (by on average 0.36 percentage points in patients with type II diabetes) (14,36). It is important to bear in mind that even a slight decrease in HbA1c may bring significant public health benefits, e.g. in terms of microvascular complications and mortality (37). The research evidence on the effect of periodontal treatment on patients with type 1 diabetes is so far inconclusive (14).
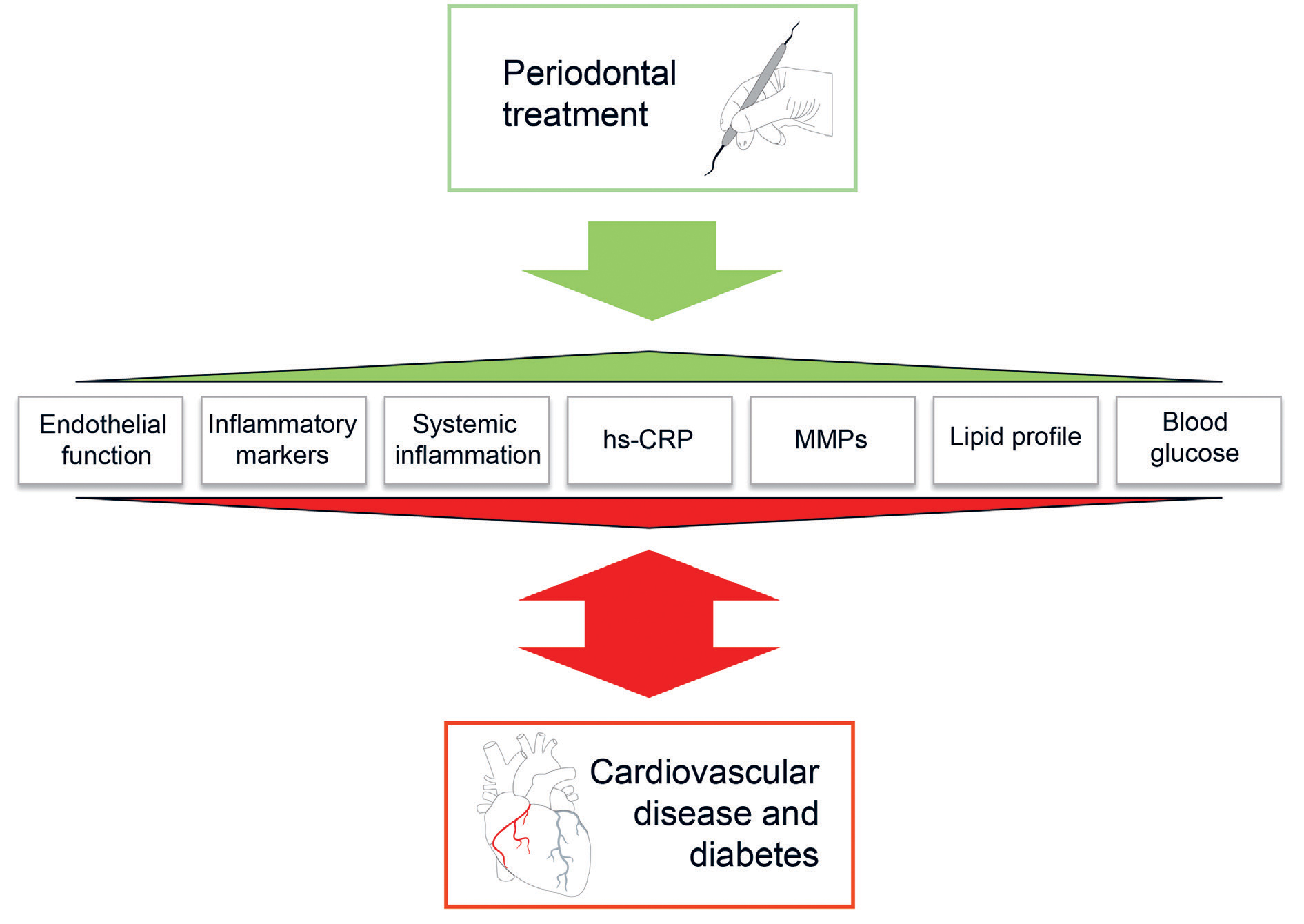
Figure 2. The beneficial effects of periodontal treatment on the markers of cardiovascular diseases and diabetes.
The Parogene study as an example of the association between periodontitis and CVD
Worldwide, there are few patient studies investigating in detail both the degree of atherosclerosis and the clinical, microbiological and radiological status of the mouth. The Finnish Parogene included 506 subjects with coronary artery disease in whom the degree of disease severity had been investigated with coronary artery angiography performed due to heart symptoms (38). The subjects underwent a clinical oral examination and panoramic radiography, and periodontal pathogens from saliva and subgingival plaque samples were analysed. The results showed that radiologically confirmed alveolar bone loss, the number of missing teeth and clinically detected inflammation of the perodontium, particularly deepened periodontal pockets, were associated with the degree of severity of stable as well as unstable coronary artery disease. In microbiological analyses, one of the risk pathogens for the development of periodontitis, Aggregatibacter actinomycetemcomitans, was particularly associated with coronary artery disease. The number of bacteria in the saliva and systemic antibodies to this pathogen (39), subgingival bacterial finding (40) as well as specific serotypes of A. actinomycetemcomitans in the saliva (18) were associated with elevated risk of coronary artery disease and degree of disease severity. The role of the immunological response induced by the LPS of the bacteria and periodontal pathogens in mediating the association between periodontitis and heart disease was also observed in the Parogene study (21,41). Future follow-up studies will show whether long-term exposure to marginal periodontitis or other oral infections has an effect on the course or outcome of heart disease.
Summary for clinical practice
– Evidence shows that periodontal treatment has beneficial effects on general health.
– Prevention of periodontal disease, identification of high-risk individuals as well as diagnosis and treatment of periodontitis, particularly in the early stages of disease, cardiovascular heart health as well.
– Keeping the oral cavity free of infections is particularly important in patients at high risk. To date, there is no recommendation concerning recall interval or more frequent maintenance in patients diagnosed with cardiovascular disease such as coronary artery disease. It is likely that in the future, a need for such a recommendation will arise.
– There is already robust evidence of the association between periodontitis and general health. The effect of other oral infections on cardiovascular health requires further study.
References
Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesäniemi YA, Syrjälä SL, Jungell PS, Isoluoma M, Hietaniemi K, Jokinen MJ. Association between dental health and acute myocardial infarction. BMJ. 1989; 298: 779–81.
Syrjänen J, Peltola J, Valtonen V, Iivanainen M, Kaste M, Huttunen JK. Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med. 1989; 225: 179–84.
Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC Jr, Baddour LM; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation 2012; 125: 2520–44.
Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965; 58: 295–300.
Janket SJ, Qvarnstrom M, Meurman JH, Baird AE, Nuutinen P, Jones JA. Asymptotic dental score and prevalent coronary heart disease. Circulation 2004; 109: 1095–100.
Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C. Poor oral health is associated with coronary heart disease and elevated systemic inflammatory and haemostatic factors. J Clin Periodontol. 2004; 31: 25–9.
Pussinen PJ, Paju S, Koponen J, Viikari JSA, Taittonen L, Laitinen T, Burgner DP, Kähönen M, Hutri-Kähönen N, Raitakari OT, Juonala M. Association of Childhood Oral Infections With Cardiovascular Risk Factors and Subclinical Atherosclerosis in Adulthood. JAMA Netw Open. 2019; 2(4): e192 523.
Fedele S, Sabbah W, Donos N, Porter S, D’Aiuto F. Common oral mucosal diseases, systemic inflammation, and cardiovascular diseases in a large cross-sectional US survey. Am Heart J. 2011; 161: 344–50.
Osmenda G, Maciag J, Wilk G, Maciag A, Nowakowski D, Loster J, Dembowska E, Robertson D, Guzik T, Czesnikiewicz-Guzik M. Treatment of denture-related stomatitis improves endothelial function assessed by flow-mediated vascular dilation. Arch Med Sci. 2017; 13: 66–74.
Lusis AJ. Atherosclerosis. Nature 2000; 407: 233–41.
Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2 000 2013; 62(1): 59–94.
Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors – tobacco smoking. J Clin Periodontol. 2005; 32 Suppl 6: 180–95.
Aarabi G, Zeller T, Seedorf H, Reissmann DR, Heydecke G, Schaefer AS, Seedorf U. Genetic Susceptibility Contributing to Periodontal and Cardiovascular Disease. J Dent Res. 2017; 96: 610–7.
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018; 45: 138–49.
Munz M, Richter GM, Loos BG, Jepsen S, Divaris K, Offenbacher S, Teumer A, Holtfreter B, Kocher T, Bruckmann C, Jockel-Schneider Y, Graetz C, Munoz L, Bhandari A, Tennstedt S, Staufenbiel I, van der Velde N, Uitterlinden AG, de Groot LCPGM, Wellmann J, Berger K, Krone B, Hoffmann P, Laudes M, Lieb W, Franke A, Dommisch H, Erdmann J, Schaefer AS. Genome-wide association meta-analysis of coronary artery disease and periodontitis reveals a novel shared risk locus. Sci Rep. 2018; 8: 13 678.
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015; 15: 30–44.
Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2018; 72: 2071–81.
Pietiäinen M, Kopra KAE, Vuorenkoski J, Salminen A, Paju S, Mäntylä P, Buhlin K, Liljestrand JM, Nieminen MS, Sinisalo J, Hyvärinen K, Pussinen PJ. A. actinomycetemcomitans serotypes associate with periodontal and coronary artery disease status. J Clin Periodontol. 2018; 45: 413–21.
Purwar P, Khan MA, Mahdi AA, Pandey S, Singh B, Dixit J, Sareen S. Salivary and serum leptin concentrations in patients with chronic periodontitis. J Periodontol. 2015; 86: 588–94.
Kebschull M, Demmer RT, Papapanou PN. «Gum bug, leave my heart alone!» – epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010; 89: 879–902.
Liljestrand JM, Paju S, Buhlin K, Persson GR, Sarna S, Nieminen MS, Sinisalo J, Mäntylä P, Pussinen PJ. Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J Clin Periodontol. 2017; 44: 784–92.
Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative Stress in Oral Diseases: Understanding Its Relation with Other Systemic Diseases. Front Physiol. 2017; 8: 693.
Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol. 2013; 40: S51–69.
Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, Palosuo T, Alfthan G, Asikainen S. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004; 45: 139–47.
Nepomuceno R, Pigossi SC, Finoti LS, Orrico SRP, Cirelli JA, Barros SP, Offenbacher S, Scarel-Caminaga RM. Serum lipid levels in patients with periodontal disease: A meta-analysis and meta-regression. J Clin Periodontol. 2017; 44:1192–207.
Kato T, Yamazaki K, Nakajima M, Date Y, Kikuchi J, Hase K, Ohno H, Yamazaki K. Oral Administration of Porphyromonas gingivalis Alters the Gut Microbiome and Serum Metabolome. mSphere 2018; 3: e00 460–18.
Nibali L, D’Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol. 2007; 34: 931–7.
Gomes-Filho IS, Freitas Coelho JM, da Cruz SS, Passos JS, Teixeira de Freitas CO, Aragão Farias NS, Amorim da Silva R, Silva Pereira MN, Lima TL, Barreto ML. Chronic periodontitis and C-reactive protein levels. J Periodontol. 2011; 82: 969–78.
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002; 347(20): 1557–65.
Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007; 356: 911–20.
Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D’Aiuto F, Kastelein JJ, Loos BG. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta‐analysis. J Clin Periodontol. 2014; 41: 70–9.
Orlandi M, Suvan J, Petrie A, Donos N, Masi S, Hingorani A, Deanfield J, D’Aiuto F. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014; 236: 39–46.
Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology study. J Am Heart Assoc. 2013; 2: e000 254.
Holmlund A, Lampa E, Lind L. Poor Response to Periodontal Treatment May Predict Future Cardiovascular Disease. J Dent Res. 2017; 96: 768–73.
Li C, Lv Z, Shi Z, Zhu Y, Wu Y, Li L, Iheozor-Ejiofor Z. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2017; 11: CD009 197.
D’Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, Masi S, Tsakos G, Hurel S, Hingorani AD, Donos N, Deanfield JE; TASTE Group. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018; 6: 954–65.
UK Prospective Diabetes Study UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–53.
Buhlin K, Mäntylä P, Paju S, Peltola JS, Nieminen MS, Sinisalo J, Pussinen PJ. Periodontitis is associated with angiographically verified coronary artery disease. J Clin Periodontol. 2011; 38: 1007–14.
Hyvärinen K, Mäntylä P, Buhlin K, Paju S, Nieminen MS, Sinisalo J, Pussinen PJ. A common periodontal pathogen has an adverse association with both acute and stable coronary artery disease. Atherosclerosis. 2012; 223: 478–84.
Mäntylä P, Buhlin K, Paju S, Persson R, Nieminen MS, Sinisalo J, Pussinen PJ. Subgingival A. actinomycetemcomitans associates with the risk of coronary artery disease. J Clin Periodontol. 2013; 40: 583–90.
Liljestrand JM, Paju S, Pietiäinen M, Buhlin K, Persson GR, Nieminen MS, Sinisalo J, Mäntylä P, Pussinen PJ. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis. 2018; 268: 177–84.
Corresponding author: Aino Salminen, aino.m.salminen@helsinki.fi
The article has been peer reviewed.
Accepted for publication 28 May 2019.
Salminen A, Kopra E, Lahdentausta L, Liljestrand J, Paju S. Association between oral infections and cardiovascular diseases. Nor Tannlegeforen Tid. 2020; 130: 122–7
Keywords: cardiovascular disease, atherosclerosis, inflammation, periodontitis
MeSH: Munn, tenner og svelg; Hjerte- og karsykdommer; Infeksjon; Kausal sammenheng