The interrelationship of periodontitis and rheumatoid arthritis
Summary
Rheumatoid arthritis (RA) and periodontitis are both common chronic inflammatory diseases and may associate bidirectionally. The diseases show several similarities in their pathogenesis i.e. inflammatory mediators, cytokines and proteolytic enzymes are largely similar, with a tissue degrading profile in the two conditions. Soft as well as hard tissues are affected. Both are multifactorial disorders, influenced by a combination of host, lifestyle (smoking), environmental and genetic components, and both have a cyclic nature, fluctuating between chronic and acute periods. The clinical course of periodontitis in RA patients has been reported as more severe independent of age, gender, ethnicity, or smoking history, and RA patients suffer from increased tooth loss and attachment loss compared with controls.
Periodontitis is a plaque-induced inflammation of the periodontal tissues causing loss of alveolar bone and periodontal attachment. The periodontal pathogen Porphyromonas gingivalis has been hypothesized to account for a possible link between the diseases through citrullination.
An increased prevalence of periodontitis in RA patients has been shown in several studies. However, the results are conflicting, and a causal relationship has not been revealed. A possible mutual effect of treatment is not ascertained. For future studies, it may be valuable to examine subgroups of RA patients, paying more attention to medication and confounders.
Headlines
An increased prevalence and severity of periodontitis in RA patients has been reported in several studies
RA and periodontitis have many pathological features in common, of which inflammation and bone destruction are the most prominent
Risk factors in both conditions are smoking and genetic susceptibility
A possible mutual effect of treatment of the diseases is not ascertained
There is currently limited evidence to support that periodontitis is a risk factor for RA
Introduction
The idea of a possible association between rheumatoid arthritis (RA) and periodontitis is far from new (1). Already Hippocrates, 400 years B.C., suggested that pulling teeth could treat arthritis. In 1918 Sturridge reported an RA patient with periodontitis whom he treated by «removing every particle of calculus and polishing the root surfaces», and «at the same time sterilized the affected tissues by zinc ionization». He declared that the periodontal treatment improved both conditions, and he was convinced of a causal relationship between periodontitis and RA. Today, a causal relationship is a matter of discussion, and possible biological mechanisms that could explain an association are the subject of extensive investigation as recently reviewed (2).
Rheumatoid arthritis
RA is a chronic, inflammatory, autoimmune disease, primarily involving the joints with degradation and deformity, swelling and pain (3). The prevalence of RA in Western countries is 0.5–1.0 % in Caucasians, but differs between ethnicities. Autoantibodies to immunoglobulin G (rheumatoid factor, RF) and antibodies to citrullinated peptides (anti-citrullinated protein antibodies, ACPAs), are found in 70–80 % of RA-patients, thereby being characteristic markers for RA, the specificity being as high as 85 % and 98 %, respectively (4, 5). Even though autoantibodies are an important feature of RA (seropositive RA), some individuals are negative for these autoantibodies (seronegative RA), and in addition, 2–5 % of the healthy population have ACPAs. RA is a complex disease, and environmental factors may trigger the disorder in genetically susceptible individuals. The pathogenic mechanisms involved in RA, as well as the clinical presentation, vary across different disease stages and between individuals. Risk factors include genetics, epigenetics, gender, lifestyle and microbiota (6).
Periodontitis
Periodontitis is a chronic inflammatory disease characterized by progressive destruction of tooth supporting tissues. Persisting biofilm and its harmful bacterial products cause an inflammatory reaction in the host. In susceptible individuals, this reaction includes activation of immune cells and release of inflammatory mediators resulting in tissue degradation. The course of periodontitis depends on several individual characteristics including genetic and environmental factors, first of all lifestyle.
Comorbidity
A number of studies have demonstrated an increased susceptibility among RA patients of acquiring advanced periodontitis compared to individuals without RA (7–10). The available studies are small and hampered by varying diagnostic criteria for both diseases. Other studies provide weaker evidence of an association between the diseases (11–13). A systematic review showed that seven of ten case-control studies found more attachment loss in RA patients and five of seven studies found significantly increased tooth loss compared to controls (14). Moreover, a meta-analysis demonstrated that the weighted mean differences between RA patients and controls were statistically significant for both periodontal attachment loss and tooth loss. The findings above have been further supported by two additional cross-sectional studies (15, 16). In another recent meta-analysis, RA patients were reported to have a 13 % greater risk of periodontitis compared to the non-RA cohort, the range being 4–13 % (15). However, current smoking status was not commented on or adjusted for in the studies included in the review. Seropositivity for RF and/or ACPAs have been associated with higher prevalence of periodontitis (16, 17). Also, ACPA-positive RA patients appear more frequently to have periodontitis than do osteoarthritis patients (16). In conclusion, while the studies demonstrate that the periodontal status is worse in RA patients compared to controls (14, 18), there is currently limited evidence to support that periodontitis is a risk factor for RA (19).
Pathogenic similarities
RA and periodontitis are both common chronic inflammatory diseases, and may associate bidirectionally. The diseases show several similarities in their pathogenesis (20), i.e. inflammatory mediators, cytokines and proteolytic enzymes are largely similar, with a tissue degrading profile in the two conditions. Soft as well as hard tissues are affected, and both are multifactorial disorders, influenced by a combination of host, lifestyle, environmental and genetic components.
Genetics
RA has a strong genetic component. From twin studies it has been estimated that the heritability of RA in patients who are positive for ACPAs is about 60 % (21), being lower for seronegative disease. Identical twins show a disease concordance of 12–15 %, indicating an important role of non-coding factors in susceptibility.
Specific class II human leukocyte antigen (HLA) loci show a very strong association with RA, and the alleles HLA DRB1*01 and HLA DRB1*04 are significantly associated with the risk of developing the disease. Genetic differences between ACPA-positive and -negative RA patients have been detected, and the two subgroups of RA patients are considered by many investigators to be two distinct disease entities. Genetic factors also influence the severity of RA. Likewise, there is a statistically significant genetic variance for both severity and extent of periodontitis, and genetic factors have been estimated to account for approximately 50 % of the attachment loss in adult periodontitis patients (22). Interestingly, HLA DRB1*04 alleles are risk factors for severe and rapidly progressive periodontal disease (23, 24).
Smoking
Cigarette smoking is one of the most established risk factors for both RA and periodontitis and has been associated with increased disease severity and lower rates of disease remission in both diseases (25, 26). Interestingly, in RA patients, smoking is believed to promote the secretion of peptidylarginine deiminase (PAD) from leukocytes, thereby probably promoting the process of citrullination (27).
A meta-analysis has described doubling of the risk of acquiring RA in current smokers with a 20-pack-year history of tobacco use, compared to nonsmokers (28). Another recent meta-analysis found that smoking increases the risk of periodontitis by 85 % (risk ratio=1.85, 95 % CI=1.5, 2.2) (29). Severe periodontitis was found in 18.9 % of present smokers, in 9.5 % of former smokers, but only in 5.5 % of non-smokers. The magnitude of therapeutic effect is compromised in smokers compared with non-smokers (30).
The highest risk of periodontitis in established RA has been observed among seropositive current smokers, especially those double positive for RF and ACPA (OR 1.9, 95 % CI 1.7–2.1) (31, 32). Current smoking ACPA-positive men had the highest prevalence (OR 2.9, 95 % CI 1.6–5.3) of periodontitis (31). Dose and duration of smoking is significant for the risk of periodontal disease as well as RA, and smoking cessation decreases the risk (32).
Microbial and immunological aspects
A possible role of Porphyromonas gingivalis in RA?
The inflammatory response in RA resembles the response observed in chronic infections, justifying search for an infectious component, which could initiate or trigger RA. Although bacterial and viral infections have been hypothesized as triggers in initiation of RA, and patients often relate the onset of their symptoms to a prior infection, studies have failed to demonstrate a specific organism to be responsible for the disease.
PAD enzymes are considered important for disease progression in ACPA-positive RA (33). About a decade ago, it was discovered that the enzyme from P. gingivalis (PPAD), equivalent to host-derived human PAD enzymes, has the ability to citrullinate both bacterial and human proteins (3, 4, 34) (Figure 1). Since P. gingivalis is considered an essential pathogen in the development of periodontitis, this finding rekindled the interest to search for a possible relationship between RA and periodontitis.
In addition to the above described PPAD involved in citrullination, bacterial enolase may also be citrullinated by human PADs thereby possibly acting as antigens in RA patients (35).
Accordingly, P. gingivalis has been proposed as a causal link between RA and periodontitis and as responsible for triggering and/or driving autoimmunity and autoimmune disease in the ACPA-positive subset of RA patients (36). Although a role of P. gingivalis has been further substantiated by the finding of ACPAs in periodontal tissue (37), its role in RA remains to be further elucidated. An increased expression of ACPAs in serum of RA patients in the presence of P. gingivalis has been reported (16), but the finding was in contrast to that of a previous study (38). Furthermore, it is still not known if subversion of host immune response by P. gingivalis is a critical event, and the impact on RA of other bacteria in the biofilm is not clarified.
P. gingivalis has many virulence factors including the gingipain family of proteases, lipopolysaccharides, and fimbriae (39, 40). Antibodies to the P. gingivalis virulence factor, arginine gingipain type B (RgpB), have been reported significantly elevated in patients with periodontitis compared to controls without periodontitis, in RA patients compared to non-RA controls, and in ACPA-positive RA patients compared to ACPA-negative RA patients (41). It is noteworthy that the association between elevated anti-RgpB levels and RA was stronger than the association between smoking and RA.
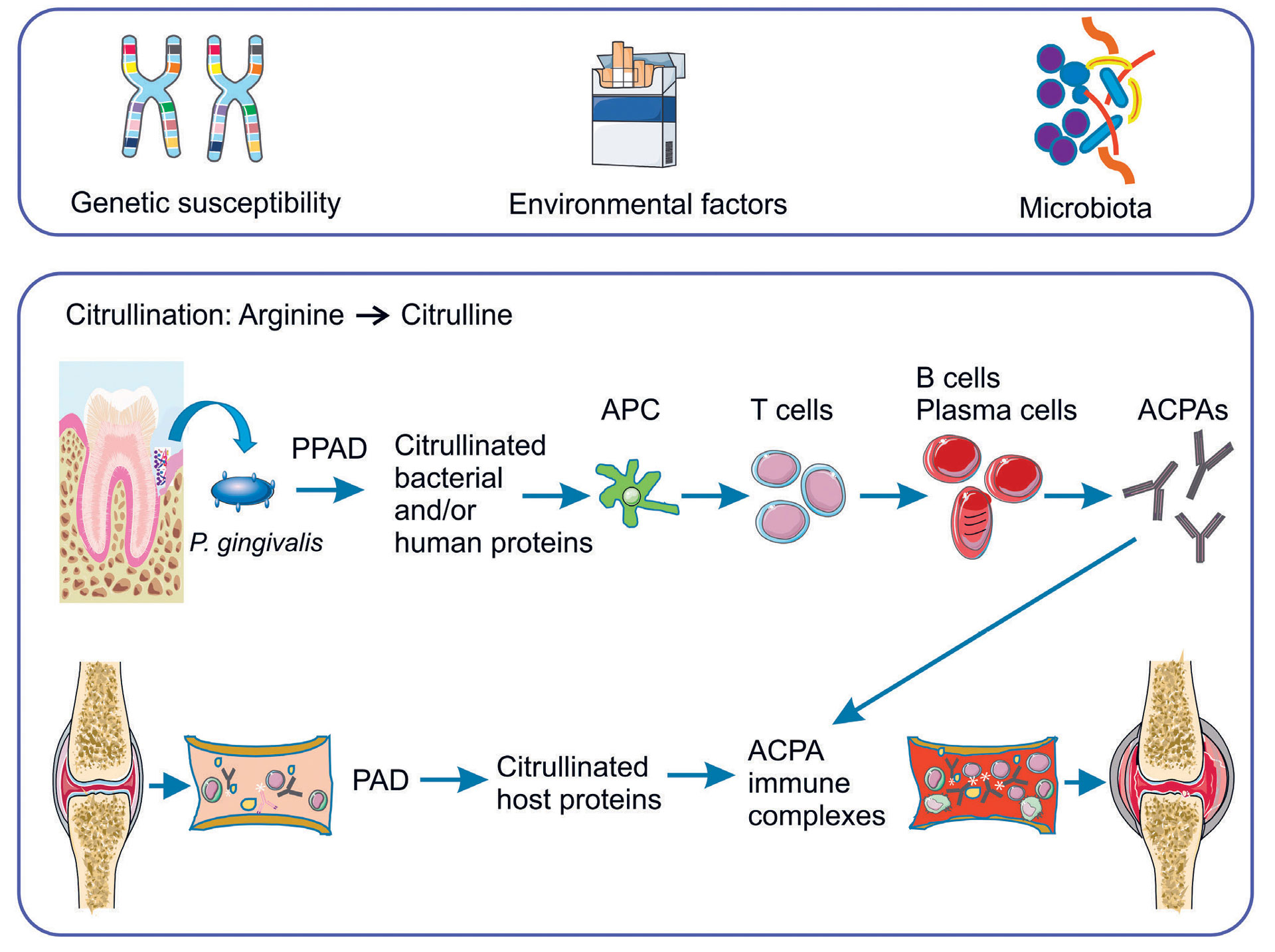
Figure 1. Risk factors and potential links between rheumatoid arthritis and periodontitis. P. gingivalis; Porphyromonas gingivalis. PPAD; peptidylarginine deiminase from P. gingivalis, PAD; peptidylarginine deiminase, APC; antigen presenting cell, ACPAs; anti-citrullinated protein antibodies. Parts of the figure were designed using Servier Medical Art (https: //smart.servier.com).
Other possible links of periodontal pathogens with RA
A number of studies have reported elevated serum antibodies in RA patients to bacteria associated with periodontitis, including P. gingivalis, Prevotella intermedia and Tannerella forsythia (42, 43). Importantly, DNA from P. gingivalis, P. intermedia, T. forsythia, T. denticola and Fusobacterium nucleatum has been identified in synovial fluid of RA patients (43–46), but no viable bacteria have been recognized so far. A possible source of bacterial DNA in the joint could originate from sources elsewhere including the blood stream.
Recently, it was shown that Aggregatibacter actinomycetemcomitans, which is another important periodontal pathogen, induces hypercitrullination in neutrophils through the activity of the pore-forming toxin leukotoxin-A (LtxA) (47), and A. actinomycetemcomitans was suggested as a candidate bacterial trigger of autoimmunity in RA.
In humans, the RA-associated subgingival microbiome is substantially different from that of healthy controls (48). Anaerobic species are enriched in the oral microbiota of RA patients, whereas health associated genera are reduced. Successful RA treatment with anti-inflammatory drugs partially reverses the oral microbial dysbiosis (49). A recent study investigated the bacterial component of the subgingival microbiome in RA patients with different periodontal conditions and related these findings to RA disease activity and periodontal health status of these individuals (50). The subgingival microbiome of subjects with active RA disease differed from those with the disease in remission. Furthermore, it was found that in RA patients with active disease, anti-inflammatory medication as part of RA therapy was associated with better oral health status and a more health related subgingival microbiome compared to that of RA patients in remission, especially among those who were current smokers (50). The microbial composition of subgingival plaque differed between no/mild and severe periodontitis in RA patients (50), which was recently confirmed by others (51), who also found the severe form of periodontitis to be significantly associated with ACPA positivity and increased levels of systemic and oral inflammatory mediators (51).
Animal studies
Several animal studies support the interactions of RA with periodontitis. Arthritis-induced alveolar bone loss in mice is associated with qualitative and quantitative changes in the composition of the oral microbiota, which is in alignment with findings in humans mentioned above (52).
Moreover, P. gingivalis induced inflammation has been shown to aggravate the course of RA in experimental animal models. Pre-existing inflammation caused by subcutaneous implantation of P. gingivalis in rats promoted development of arthritis (53). Furthermore, pre-existing periodontitis induced by oral infection of P. gingivalis in combination with F. nucleatum generated antibodies against citrullinated peptides derived from PPAD in rats (54). Very recently, it was shown that periodontitis, induced by oral exposure to P. gingivalis, triggered seropositive arthritis with systemic inflammation and bone erosions (55).
Inflammation and cytokine network
In both RA and periodontitis, activation of the innate and the acquired immune system leads to secretion of the proinflammatory cytokines, interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNF-α), lipid mediators including prostaglandin E2 (PGE2), and the tissue destructive enzymes matrix metalloproteinases (MMPs). Complex inflammatory signals and cytokine networks collectively regulate osteoclastogenesis. Disruption of the balance between osteoblast and osteoclast activities by bacterial products and/or inflammatory cytokines causes inflammation-induced bone loss (56), which is characteristic of both diseases. Moreover, a systemic low-grade inflammation induced by periodontitis may worsen the inflammatory reactions in the joints of RA patients and vice versa (18). Similarly, elevated levels of inflammatory markers have been found in patients with generalized aggressive periodontitis and in patients with RA (10). Additionally, dysregulation of immunoinflammatory responses has been found in both diseases (8, 57–59). For further details, see (60).
Effect of periodontal treatment
The effect of periodontal treatment in RA patients has been evaluated in a number of studies, as previously reviewed (61). Although small and with limited follow-up period, the available studies suggest that non-surgical periodontal treatment may reduce clinical symptoms and biomarkers of active RA (61). One of the problems is that the patients receive various types of anti-rheumatic drugs, which complicates the formation of uniform groups of patients. This is evident in a study of 40 patients with severe to moderate RA, who received anti-rheumatic drugs alone or combined with anti-TNF-α. Six weeks after non-surgical periodontal treatment, a positive effect on symptoms and signs of RA was found (62). Another study on the effect of full-mouth scaling and root-planing in RA patients resulted in reduced erythrocyte sedimentation rate (ESR) after three months, but showed no effect on disability or IgM-RF (63).
A pathogenic pathway common to both chronic periodontitis and RA is the excessive degradation of collagen-rich tissues. The collagenolytic MMPs are major contributors to this degradation in gingiva, periodontal ligament, and alveolar bone in periodontitis, and bone, cartilage, and other periarticular tissues in RA. Accordingly, the effect of MMP inhibitor drugs on tissue destruction has been investigated, and they appear to have similar outcome on RA and periodontitis. This effect is synergistically enhanced in combination with anti-inflammatory drug treatment, and supposed to be due to reduced systemic inflammation (18). Nevertheless, most of these studies are conducted in animal models, and the results must be interpreted with caution.
Oral hygiene instructions and non-surgical treatment reduce clinical signs and symptoms in active RA and all clinical periodontal parameters compared to RA patients who do not receive periodontal treatment (62, 64).
There are few studies available investigating the influence of RA on the outcome of dental implant therapy. A systematic review reported success rates in the range of 93.8 % and 96.1 % (65). None of the studies found significant correlations between RA and implantation success. However, the review included only few publications and insufficient data for a conclusion, and further detailed studies are needed.
Conclusion
The majority of studies on the association of periodontitis with RA have confirmed an interrelationship of the two diseases including clinical and immunological similarities. However, further research is required to elucidate the mechanisms linking the two diseases. Investigations on the capability of oral bacteria to elicit an autoimmune response and the associated molecular mechanisms are called for. In addition, there is a need for longitudinal and large cohort studies on the effect on RA of periodontal treatment.
References
Sturridge E. Case of Rheumatoid Arthritis treated by Ionization of Periodontal Membrane. Proc R Soc Med. 1918; 11(Odontol Sect): 112–4.
Araujo VM, Melo IM, Lima V. Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediators Inflamm. 2015; 2015: 259074.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010; 376(9746): 1094–108.
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000; 43(1): 155–63.
Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007; 146(11): 797–808.
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018; 4: 18001.
Kasser UR, Gleissner C, Dehne F, Michel A, Willershausen-Zonnchen B, Bolten WW. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997; 40(12): 2248–51.
Mercado F, Marshall RI, Klestov AC, Bartold PM. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000; 27(4): 267–72.
Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001; 72(6): 779–87.
Havemose-Poulsen A, Westergaard J, Stoltze K, Skjodt H, Danneskiold-Samsoe B, Locht H, et al. Periodontal and hematological characteristics associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2006; 77(2): 280–8.
de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008; 35(1): 70–6.
Arkema EV, Karlson EW, Costenbader KH. A prospective study of periodontal disease and risk of rheumatoid arthritis. J Rheumatol. 2010; 37(9): 1800–4.
Demmer RT, Molitor JA, Jacobs DR, Jr., Michalowicz BS. Periodontal disease, tooth loss and incident rheumatoid arthritis: results from the First National Health and Nutrition Examination Survey and its epidemiological follow-up study. J Clin Periodontol. 2011; 38(11): 998–1006.
Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013; 92(5): 399–408.
de Smit M, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer E, van Winkelhoff AJ. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012; 14(5): R222.
Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014; 66(5): 1090–100.
Beyer K, Lie SA, Kjellevold M, Dahl L, Brun JG, Bolstad AI. Marine omega-3, vitamin D levels, disease outcome and periodontal status in rheumatoid arthritis outpatients. Nutrition. 2018; 55–56: 116–24.
Payne JB, Golub LM, Thiele GM, Mikuls TR. The Link Between Periodontitis and Rheumatoid Arthritis: A Periodontist’s Perspective. Curr Oral Health Rep. 2015; 2: 20–9.
Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013; 84(4 Suppl): S8-S19.
Cantley MD, Haynes DR, Marino V, Bartold PM. Pre-existing periodontitis exacerbates experimental arthritis in a mouse model. J Clin Periodontol. 2011; 38(6): 532–41.
MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000; 43(1): 30–7.
Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE, et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000; 71(11): 1699–707.
Bonfil JJ, Dillier FL, Mercier P, Reviron D, Foti B, Sambuc R, et al. A «case control» study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO). J Clin Periodontol. 1999; 26(2): 77–84.
Firatli E, Kantarci A, Cebeci I, Tanyeri H, Sonmez G, Carin M, et al. Association between HLA antigens and early onset periodontitis. J Clin Periodontol. 1996; 23(6): 563–6.
Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006; 54(1): 38–46.
Sokolove J, Wagner CA, Lahey LJ, Sayles H, Duryee MJ, Reimold AM, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016; 55(11): 1969–77.
Catrina AI, Joshua V, Klareskog L, Malmstrom V. Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev. 2016; 269(1): 162–74.
Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010; 69(1): 70–81.
Leite FRM, Nascimento GG, Scheutz F, Lopez R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am J Prev Med. 2018; 54(6): 831–41.
Kotsakis GA, Javed F, Hinrichs JE, Karoussis IK, Romanos GE. Impact of cigarette smoking on clinical outcomes of periodontal flap surgical procedures: a systematic review and meta-analysis. J Periodontol. 2015; 86(2): 254–63.
Eriksson K, Nise L, Alfredsson L, Catrina AI, Askling J, Lundberg K, et al. Seropositivity combined with smoking is associated with increased prevalence of periodontitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2018; 77(8): 1236–8.
Hedstrom AK, Stawiarz L, Klareskog L, Alfredsson L. Smoking and susceptibility to rheumatoid arthritis in a Swedish population-based case-control study. Eur J Epidemiol. 2018; 33(4): 415–23.
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998; 101(1): 273–81.
Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004; 28(6): 311–8.
Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008; 58(10): 3009–19.
Bingham CO, 3rd, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 2013; 25(3): 345–53.
Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, Bartold PM. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodontal Res. 2013; 48(2): 252–61.
Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012; 64(10): 3083–94.
Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012; 333(1): 1–9.
Potempa J, Pike R, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem. 1997; 378(3–4): 223–30.
Kharlamova N, Jiang X, Sherina N, Potempa B, Israelsson L, Quirke AM, et al. Antibodies to Porphyromonas gingivalis Indicate Interaction Between Oral Infection, Smoking, and Risk Genes in Rheumatoid Arthritis Etiology. Arthritis Rheumatol. 2016; 68(3): 604–13.
Ogrendik M, Kokino S, Ozdemir F, Bird PS, Hamlet S. Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. MedGenMed. 2005; 7(2): 2.
Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009; 9(1): 38–42.
Martinez-Martinez RE, Abud-Mendoza C, Patino-Marin N, Rizo-Rodriguez JC, Little JW, Loyola-Rodriguez JP. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 2009; 36(12): 1004–10.
Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006; 24(6): 656–63.
Temoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012; 18(3): 117–21.
Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016; 8(369): 369ra176.
Scher JU, Bretz WA, Abramson SB. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors? Curr Opin Rheumatol. 2014; 26(4): 424–9.
Graves DT, Correa JD, Silva TA. The Oral Microbiota Is Modified by Systemic Diseases. J Dent Res. 2018: 22034518805739.
Beyer K, Zaura E, Brandt BW, Buijs MJ, Brun JG, Crielaard W, et al. Subgingival microbiome of rheumatoid arthritis patients in relation to their disease status and periodontal health. PLoS One. 2018; 13(9): e0202278.
Eriksson K, Fei G, Lundmark A, Benchimol D, Lee L, Hu YOO, et al. Periodontal Health and Oral Microbiota in Patients with Rheumatoid Arthritis. J Clin Med. 2019; 8(5).
Correa JD, Saraiva AM, Queiroz-Junior CM, Madeira MF, Duarte PM, Teixeira MM, et al. Arthritis-induced alveolar bone loss is associated with changes in the composition of oral microbiota. Anaerobe. 2016; 39: 91–6.
Bartold PM, Marino V, Cantley M, Haynes DR. Effect of Porphyromonas gingivalis-induced inflammation on the development of rheumatoid arthritis. J Clin Periodontol. 2010; 37(5): 405–11.
Eriksson K, Lonnblom E, Tour G, Kats A, Mydel P, Georgsson P, et al. Effects by periodontitis on pristane-induced arthritis in rats. J Transl Med. 2016; 14(1): 311.
Courbon G, Rinaudo-Gaujous M, Blasco-Baque V, Auger I, Caire R, Mijola L, et al. Porphyromonas gingivalis experimentally induces periodontis and an anti-CCP2-associated arthritis in the rat. Ann Rheum Dis. 2019; 78(5): 594–9.
Liu YC, Lerner UH, Teng YT. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000. 2010; 52(1): 163–206.
Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. 2005; 76(11 Suppl): 2066–74.
Havemose-Poulsen A, Sorensen LK, Stoltze K, Bendtzen K, Holmstrup P. Cytokine profiles in peripheral blood and whole blood cell cultures associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2005; 76(12): 2276–85.
Kaur S, White S, Bartold M. Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review. JBI Libr Syst Rev. 2012; 10(42 Suppl): 1–12.
Holmstrup P, Nielsen CH. Linkage between periodontal disease and rheumatoid arthritis. In: Pedersen A, editor: Oral Infections and General Health. Switzerland. Springer International Publishing; 2016, 45–51.
Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2014; 44(2): 113–22.
Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, Panneerselvam A, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009; 80(4): 535–40.
Ribeiro J, Leao A, Novaes AB. Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol. 2005; 32(4): 412–6.
Pinho Mde N, Oliveira RD, Novaes AB, Jr., Voltarelli JC. Relationship between periodontitis and rheumatoid arthritis and the effect of non-surgical periodontal treatment. Braz Dent J. 2009; 20(5): 355–64.
Guobis Z, Pacauskiene I, Astramskaite I. General Diseases Influence on Peri-Implantitis Development: a Systematic Review. J Oral Maxillofac Res. 2016; 7(3): e5.
Corresponding author: Anne Isine Bolstad, Department of Clinical Dentistry, Årstadveien 19, 5009 Bergen, Norway. E-mail: anne.bolstad@uib.no
This paper has been peer reviewed.
Bolstad AI, Poulsen AH, Yucel-Lindberg T, Klinge B, Holmstrup P. The interrelationship of periodontitis and rheumatoid arthritis. Nor Tannlegeforen Tid. 2020; 130: 26–32
Accepted for publication 3. June 2019.
MeSH: Periodontitis; Arthritis, Rheumatoid; Comorbidity; Systemic disease; Smoking
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Bolstad AI, Havemose-Poulsen A, Yucel-Lindberg T, Klinge B, Holmstrup P. The interrelationship of periodontitis and rheumatoid arthritis. Nor Tannlegeforen Tid. 2020;131:26-32. doi:10.56373/2020-1-6