Treatment of acute dental infections
Antimicrobial resistance is globally a growing public health threat. Constrained and appropriately targeted use of antibiotics in health care could slow down the development of antibiotic resistance. Dentists are responsible for a substantial proportion of all antibiotic prescriptions, thus it is important that dentists know optimal principles and practices of using antibiotics. In Nordic countries, national guidelines have an important role as a source of up to date knowledge and expert opinion on treatment of dental infections including the use of antibiotics. The Nordic guidelines highlight that antibiotics should be prescribed only with strict indications and the use of broad-spectrum antibiotics should be avoided. At the same time, it should be kept in mind that common, localised dental infections may occasionally develop into severe life-threatening infections. Whenever antibiotic treatment is considered in addition to surgical drainage it should be started an hour before the procedure to avoid spread of the infection when manipulating infected tissues. It is essential that dentists can identify the patients with systemic infection or increased infection risk. Antibiotics are needed in severe infections and for patients with increased risk of infection complications, but primarily, acute dental infections are always treated by efficient drainage and elimination of the infection focus.
Key points | |
|---|---|
· |
Acute dental infections should primarily be treated by drainage and removal of the infection focus |
· |
Antibiotics should only be prescribed if there are signs of spreading infection and in suspicion of systemic infection or when local infection is judged not to heal with local treatment alone |
· |
Although the Nordic guidelines differ in some details they share the common feature that the base for treatment of dental infections is Pencillin V |
· |
Unnecessary use of antibiotics should be avoided because of the growing problem of antibiotic resistance |
Susceptibility of bacterial pathogens to antibiotics has significantly decreased globally over the past two decades. Increasingly many bacteria are also resistant to three or more antibiotics (multidrug resistant strains), all or nearly all current antibiotics (extensively drug resistant strains). WHO has stated that antibiotic resistance is one of the most important public health threats globally. In addition to their activity on targeted pathogens, antibiotics also have an impact on the normal microbiota by creating a selection pressure that favours resistant bacterial strains. Appropriate and constrained use of antibiotics is one of the most important ways of minimising development of drug resistance. (1) The aim of antibiotic stewardship is both to preserve the future effectiveness of antibiotics and to improve patient outcomes. Giving the right drug to the right patient at the right dose at the right time for the right duration via the right route is key in this, and antibiotic guidelines are an important tool for clinical decision-making.
Dentists prescribe a substantial proportion (7 - 11 %) of all oral antibiotics. Although many of these antibiotic prescriptions may be appropriate and decrease morbidity and mortality to odontogenic infections some are unnecessary or inappropriate (2, 3). Dentists look after patients with increasingly complex medical histories whereby it can be challenging to identify the patients that would need antibiotic therapy. Severely immunocompromised patients can lack typical symptoms of infection because of their deficient immune system and this can make it challenging to diagnose a severe infection. Correct treatment of an acute infection reduces the risk for complicated prolonged infections but will also have the benefit of minimizing the need for antibiotic treatment. The purpose of this review is to give an overview on etiology and diagnosis of local acute dental infections and their recommended treatment principles in outpatient care, and to discuss the current guidelines in the Nordic countries.
Etiology and pathogenesis of acute dental infections
Origins of dental infections
Significant proportion of head and neck infections originate from teeth. Most common sources of dental infections are apical periodontitis, pericoronitis, surgical sites, surgical tooth extraction sites and periodontitis (4,5). The most common route of infection is through the root canal: necrotic pulp becomes infected which leads to the development of apical periodontitis as the immune system is not able to eradicate the microbes in the absence of access to the root canal. The pulp tissue can be infected via various routes: dental caries, tooth fractures, open restoration margins or periodontal disease (6). Acute periodontal infections include periodontal abscess and necrotizing ulcerative gingivitis (NUG) (7). Pericoronitis, local infection of the gingiva and soft tissues adjacent to a partially erupted tooth, is frequently associated with impacted third molars. Most common postoperative complications of surgical tooth extraction are localized surgical site infections and dry socket (8). These mild, localized dental infections may in some cases lead to abscess formation and even to spreading or systemic infections.
Microbiology of dental infections
Purulent dental infections are polymicrobial in nature and usually originate from oral normal microbiota. A typical finding is a mix of strict anaerobic and facultative anaerobic bacteria with anaerobes as dominating species (9). The most common anaerobic isolates in dental abscesses are gram-negative rods, such as Prevotella, Porphyromonas and Fusobacterium species and anaerobic streptococci. Streptococcus anginosus group bacteria among viridans group streptococci are the dominating facultative anaerobic findings. Infrequently also bacterial species considered as transient colonizers (e.g. staphylococci, beta-hemolytic streptococci and enteric rods) may be found in oral infections. These transient colonizers are generally not considered as cause of infection. It is important to remember that in patients with a recent history or hospitalization and broad-spectrumantibiotics, the oral microbiome may have become significantly altered and include various more difficult to treat pathogens (10).
Complications of dental infections
Dental infections may spread locally or hematogenously and develop into severe, life-threatening, disseminated or distant site infections (11, 12). Infection tends to spread towards the lowest tissue resistance, which is dictated by anatomical constraints of the bone, muscle and fascia (figure 1). The most common locations for dental abscess formation are submandibular and sublingual spaces. In some cases, the infection can spread into deep musculofascial spaces and other vital structures such as mediastinum. In addition, locally or hematogenously spreading dental infections may cause osteomyelitis of the jaws or at a distant site. Severe complications of dental infections include respiratory obstruction, sepsis, Ludwig's angina, cervicofacial necrotizing fascitis and cavernous sinus thrombosis, which are potentially life-threatening conditions associated with substantial mortality. (13)
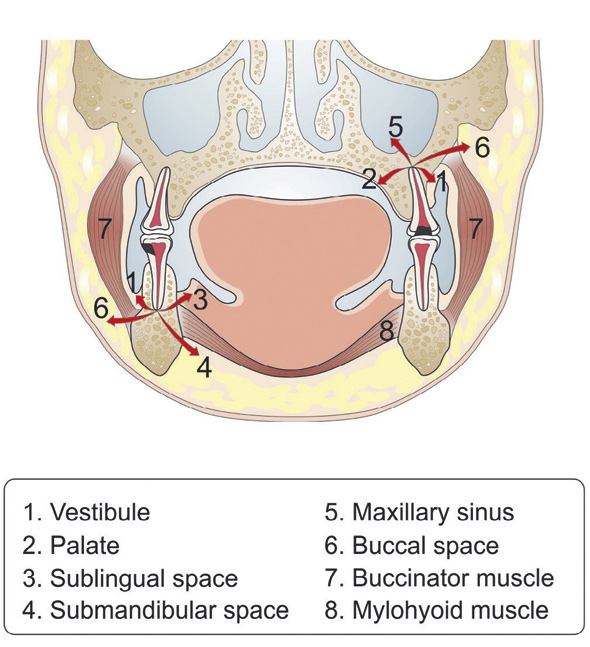
Figure 1. Possible routes for the spreading of dental infections.
Hematogeneous spread can either occur spontaneously due to the pressure caused by the unresolved infection or following invasive procedures aiming to remove the source of infection. Infective endocarditis is a rare, but life threatening distant site infection in which bacteria adhere to damaged or prosthetic heart valves, leading to cardiac lesions with bacterial growth, and to dissemination of septic emboli. Most dental procedures cause transient bacteremia which can be high in intensity particularly following invasive procedures at infected sites, but low-grade bacteremia also arises spontaneously during chewing, tooth brushing and flossing. (12)
Fully functioning immune system quickly clears low-grade bacteremia but high-grade bacteremia can be a challenge especially in immunocompromised patients. Underlying severe diseases and immunosuppressive conditions or treatments increase the risk of infection complications. In patients with dental infections, diabetes seems to associate with longer periods of hospitalization, slower resolving of infection and severe complications. In addition, elderly patients are at higher risk of developing spreading infections and infection complications. (14)
Incidence
Localised dental infections are very common but their exact incidence is not known. Based on a large systematic analysis, radiological findings of periapical radiolucencies possibly indicating dental infections are seen in 0.5 - 13.9 % of all teeth (15). Yet, the risk for severe dental infections seems to be low. Incidence of severe dental infections requiring hospital care is 1,5 - 7,2/100 000/year (14,16) and the associated mortality is 0,2/100000/year (14, 17). In severe generalized dental infections requiring intensive care, mortality has been reported to be as high as 20 - 30 %» (14, 18). The incidence of severe dental infections is higher in lower socioeconomic classes, especially in developing countries (19). This points out the preventive role of regular dental care.
Diagnosis of acute dental infections
Early and accurate diagnosis is a prerequisite for effective treatment of all dental infections and for the prevention of potential infection complications.
In clinical examination, the classic symptoms of bacterial infections, erythema, fever, oedema and pain, are often seen in acute infections of oral cavity. Depending on severity of the infection, symptoms can vary widely, from mild discomfort to severe pain, and from slight swelling to trismus and airway obstruction (17). If any symptoms of spreading or systemic infection (fever, high heart rate, malaise, altered mental status, strong physical discomfort, increasing swelling, difficulties in swallowing, breathing or speaking, rash, trismus and rapid development of symptoms) are present, the patient should be referred to a hospital emergency department without delay. The hallmarks of severe infection are presented in table 1.
Clinical examination |
Primary health care |
Hospital care indicated |
|---|---|---|
Anamnesis |
Previously healthy |
Illness or medication that predisposes to infection complications |
Clinical status |
Normal |
Fever, high heart rate, malaise, altered mental status, strong physical discomfort |
Inspection & palpation |
Local swelling, slight erythema, heat |
Increasing swelling, difficulties in swallowing, breathing or speaking, rash |
Mouth opening |
Normal or slightly limited |
Clearly limited |
Imaging plays an important role in the identification of the infection focus. In outpatient care, conventional radiographs (orthopantomogram and periapical radiographs) are used (5). Computed tomography and ultrasound imaging are used in the diagnosis and surgical managementof severe spreading infections (20). In in-patient care of severe infections, clinical examination can be complemented with determination of leukocyte count and serum C-reactive protein (CRP) level (21).
Microbiological diagnostics
Microbiological diagnostics is not routinely required in the management of acute dental infection (22). Indications for sampling are severe dental infection, refractory infection, infections with poor response for standard treatment, dental infections of immunocompromised patients and patients with a recent heavy antibiotic treatment. Bacterial culture is the method of choice to be used in clinical practise. Culture enables identification of a broad spectrum of species as well as sensitivity testing. The sample should preferably be taken as an aspirate through disinfected mucosa or skin when possible. This way there is minimal risk for contaminating the sample by colonising bacteria from the mucosa. Also, the recovery of strict anaerobes from aspirates is better compared to swab samples (23). The transport medium used for sample transportation should support the viability of anaerobes as well as aerobes.
Prevention of acute dental infections and infection complications
Efficient prevention or early treatment of localised common dental infections would significantly reduce the need for antibiotic prescriptions. High standard of oral hygiene and regular dental care are the most important factors in the prevention of dental infections. Early and effective treatment of localized infections is vital for prevention of serious infection complications. Good oral hygiene and health can also minimize the bacteremia caused by normal oral activities as well as dental procedures. (12, 24). Educating general public on effective self-care should be one of the key priorities in dental antibiotic stewardship.
To prevent the development of generalized infections or distant site infections, all dental infection foci should be eliminated before the initiation of treatments or medications resulting in prolonged deep immunosuppression (25).
Treatment of acute dental infections
Elimination of the source of infection
The first and most important step in the treatment of acute dental infections is to eliminate the source of infection as early and effectively as possible, to achieve fast resolution of infection and to prevent infection complications (26). In localised dental infections, routine dental care is sufficient to resolve the infection. In acute apical periodontitis, the appropriate treatment is either root canal treatment or tooth extraction. The treatment of periodontal abscesses consists of mechanical debridement and drainage through gingival pocket. In NUG, the primary treatment aims at pain relief and prevention of disease progression, and it consists of careful mechanical debridement and the use of antiseptic mouth rinses. (7)
According to the literature, surgical drainage and clearance is needed in 45 - 94 % of all cases of localized dental abscess, and the indications are based on the clinical and radiological findings (27, 28). To drain the abscess, it is not recommended to make the incision to the most prominent part of the abscess, but rather further to periphery. Blunt instrument, such as hemostat or blunt scissor, is used to enter the abscess cavity. For microbiological diagnostics, a sample of the pus should be taken by aspiration. After complete evacuation of pus, the abscess cavity is rinsed with saline (together with 1,5 - 3 % hydrogen peroxide according to the Finnish guidelines). The use of a drain is recommended; one end of the drain is placed to the floor of the abscess cavity, and the other end is fixed to mucosa with sutures. The purpose of the drain is to prevent recurrence of the abscess and to maintain access to the abscess cavity for rinsing. After the surgical procedure, use of an antimicrobial mouth rinse (chlorhexidine 0,12 - 0,2 %) can be considered, and antibiotics are prescribed when needed. A close follow-up, starting on the first post-operative day, is recommended. The patient is inspected and the abscess cavity rinsed again with saline or hydrogen peroxide. If neither suppuration nor signs of recurrence of the abscess are present, the drain can be removed and the wound is left open to heal. With any sign of further pus formation, the drain can be kept in place for another day. According to the literature, early surgical intervention is effective and a reoperation is seldom needed (29).
Antibiotic therapy
Indications
Although dental or surgical elimination of the infection focus is always the cornerstone in the treatment of dental infections, adjunctive antibiotics are needed in severe and spreading infections, particularly in patients with compromised immune systems (26). The need for antibiotic treatment is individually evaluated in each case, based on symptoms and severity of infection. Antibiotic treatment is indicated in cases of rapidly spreading dental infections and if symptoms of generalized infection are present. In addition, antibiotics may be indicated in dental infections of medically compromised or immunocompromised patients, due to their increased risk of infection complications. (14, 17)
Principles of antibiotic treatment
In previously healthy, immunocompetent patients with localized dental infections, the use of antibiotics is not indicated as it does not significantly improve the treatment outcome (26). In the treatment of acute apical abscesses of previously healthy patients, antibiotics do not reduce postoperative pain or swelling following root canal treatment or incision (30). It has been shown that prophylactic antibiotics do not prevent endodontic flare-up after root canal treatment of asymptomatic teeth (31). Antibiotics are ineffective for pain relief and thus contraindicated for patients with untreated irreversible pulpitis (32). Similarly, there is no need for antibiotic therapy in the treatment of periodontal abscesses of previously healthy patients, as there is no evidence supporting the efficacy of systemic antibiotics in acute periodontal infections (7).
The antibiotic drug of choice should cover those oral bacterial species that are likely to cause complications such as spreading of infection or a distant site infection. These include oral streptococci and anaerobic gram-negative rods, such as Prevotella, Porphyromonas and Fusobacterium species (11, 33). These key findings in dental infections are usually susceptible to penicillin V, the effect and antibiotic coverage of which may be enhanced by combining with metronidazole. (34). When used, antibiotic treatment should already be started before the surgical drainage to avoid spreading of infection.
A history of antibiotic allergy and previous anaphylactic reaction are general contraindications for antibiotic drugs. A previous anaphylactic reaction to any penicillin is a contraindication for penicillin V and other beta-lactams. Tetracycline is contraindicated for pregnant and breast-feeding women, as well as for children under the age of 8 or 12 years, depending on the country-specific differences in the age recommendation. Metronidazole is contraindicated during the first trimester of pregnancy.
Discussion
National guidelines are available in all Nordic countries to aid dental practitioners in the management of dental infections. There is a consensus in these guidelines that acute dental infections should be treated primarily by prompt and proper drainage and removal of the source of infection. There is also an agreement that in addition to these dental procedures antibiotics are needed in the management of spreading or systemic infections and in patients with increased risk of infection complications (34, 35). As a basic principle, antibiotics should be prescribed only for defined indications and the use of broad-spectrum antibiotics should be avoided.
The Nordic guidelines agree on pencillin V being the mainstay of therapy for dental infections. The key similarities and differences between the national guidelines are presented in Table 2. In Sweden, metronidazole is added in case of treatment failure or severe infection or suspicion of emerging severe infection. In Norway, metronidazole is not recommended for acute infections, whereas in Denmark and Finland penicillin V is combined with metronidazole. The Finnish guideline differs from the other Nordic guidelines in that it gives guidance for treatment of immunocompromised patients and suggests lower threshold for prescribing of antibiotics for immunocompromised patients. Overall, the Nordic guidelines for treatment of acute dental infections are not significantly divergent and it appears entirely feasible to find consensus on the points that now differ between the national guidelines. To strengthen the cooperation between the Nordic countries, a shared guideline should be set as a future goal.
Similarities |
Principles of treatment |
|
Treatment primarily by surgical/dental means |
||
Antibiotics only in severe/spreading infections |
||
Avoidance of broad spectrum antibiotics |
||
Differences |
First-choice antibiotic |
Treatment of patients allergic to penicillin |
Sweden: penicillin V 1600 mg x 3, 5 - 7 days (complemented with metronidazole 400 mg x 3 if needed) |
Sweden: clindamycin 150 mg x 3 |
|
Norway: penicillin V 660 mg x 4, 5 days |
Norway: clindamycin 300 mg x 4 - 5 |
|
Denmark: penicillin V 1 million IU x 3 +metronidazole 500 mg x 3, 3 days |
Denmark: clindamycin 300 mg x 3 |
|
Finland: penicillin V 1 million IU (606 mg) x3 - 4 + metronidazole 400 mg x 3, 5 days |
Finland: cephalexin 500 mg x 3 and metronidazole 400 mg x 3 / clindamycin 300 mg x 4 if history with anaphylaxis |
Medically complex patients are increasingly common in dental outpatient setting as the population ages and there are more and more elderly dentate individuals in the population. Thus, dental practitioners often have to face the challenge of treating potentially severe dental infections in immunocompromised patients. To be able to identify and treat these patients safely, it is important for dental practitioners both to understand the medical conditions that increase patient's risk for infection complications and to raise their awareness of manifestations of oral infections in different patient populations. Most, if not all, signs of severe infection are mediated by immune responses and in immunocompromised patients even a severe infection may present with very few symptoms. As the role of the immune system is to restrict the spread of the infection by forming an abscess, infection in immunocompromised patients may spread without any obvious abscess formation. In addition, these patients have often required numerous hospital admissions and courses of broad-spectrum antibiotics and their oral microbiome is likely to have become enriched with resistant opportunistic pathogens. All this together can be a major challenge for the busy dental practitioner. Signposting the management of such patients can help to ensure better, informed decision-making, safe management and to avoid overuse of antibiotics.
As severe dental infections most often originate from common mild dental infections (caries and periodontitis), the ideal way of decreasing the use of antibiotics would be to invest heavily on maintenance of good oral health in the population. High standard of oral hygiene and frequent dental care would efficiently decrease the incidence of severe infections of dental origin. In addition, early treatment of mild localised dental infections while the patient is still healthy - without systemic predisposing factors - would decrease the incidence of infection complications and the need of using antibiotics to complement operative treatment of infection. Dentists have a key role in dental education and promotion of good oral health. Educating general public on effective self-care should be one of the key priorities in dental antibiotic stewardship.
References
WHO. Antimicrobial resistance: global report on surveillance. 2014 ISBN 978 92 4 156474 8
Halling F, Neff A, Heymann P, Ziebart T. Trends in antibiotic prescribing by dental practitioners in Germany. J Craniomaxillofac Surg. 2017 Nov; 45(11): 1854 - 59.
Preus HR, Fredriksen KW, Vogsland AE, Sandvik L, Grytten JI. Antibiotic-prescribing habits among Norwegian dentists: a survey over 25 years (1990 - 2015). Eur J Oral Sci. 2017 Aug; 125(4): 280 - 287.
Chow A. Infections of the oral cavity, neck and head. Kirjassa: Principles and practice of infectious diseases. Mandell GL, Bennett JE, Dolin R (toim.). Volume 1. 6. painos. Philadelphia: Churchill Livingstone. 2005; 787 - 802.
Richardson R, Seppänen L. Leukojen alueen syvät infektiot. Duodecim. 2010; 126: 695 - 701.
Kirkevang, Vaeth, Hörsted-Bindslev, Bahrami, Wenzel. Risk factors for developing apical periodontitis in a general population. Int Endod J. 2007; 40: 290 - 299.
Herrera D, Alonso B, de Arriba L ym. Acute periodontal lesions. Periodontol 2000, 2014; 65: 149 - 77.
Lodi G, Figini L, Sardella A ym. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst Rev. 2012; 11: CD003811.
Siqueira JF Jr, Rôças IN. Microbiology and treatment of acute apical abscesses. Clin Microbiol Rev. 2013 Apr; 26(2): 255 - 73.
Tada A, Hanada N. Opportunistic respiratory pathogens in the oral cavity of the elderly. FEMS Immunol Med Microbiol. 2010 Oct; 60(1): 1 - 17.
Parahitiyawa NB, Jin LJ, Leung WK ym. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. 2009; 22: 46 - 64.
Moreillon P, Que YA. Infective endocarditis. Lancet. 2004 Jan 10; 363(9403): 139 - 49. Review
Bali RK, Sharma P, Gaba S, Kaur A, Ghanghas P. A review of complications of odontogenic infections. Natl J Maxillofac Surg. 2015 Jul-Dec; 6(2): 136 - 43.
Lee JJ, Hahn LJ, Kao TP, Liu CH, Cheng SJ, Cheng SL, Chang HH, Jeng JH, Kok SH. Post-tooth extraction sepsis without locoregional infection-a population-based study in Taiwan. Oral Dis. 2009 Nov; 15(8): 602 - 7
Pak JG, Fayazi S, White SN. Prevalence of periapical radiolucency and root canal treatment: a systematic review of cross-sectional studies. J Endod. 2012; 38: 1170 - 6.
Seppänen L, Rautemaa R, Lindqvist C ym. Changing clinical features of odontogenic maxillofacial infections. Clin Oral Investig. 2010; 14: 459 - 65.
Seppänen L, Lauhio A, Lindqvist C ym. Analysis of systemic and local odontogenic infection complications requiring hospital care. J Infect. 2008; 57: 116 - 22.
Eisler L, Wearda K, Romatoski K ym. Morbidity and cost of odontogenic infections. Otolaryngol Head Neck Surg. 2013; 149: 84 - 8.
Agarwal, Sethi, Sethi, Mrig, Chopra. Role of socioeconomic factors in deep neck abscess: a prospective study of 120 patients. Br J Oral Maxillofac Surg. 2007; 45: 553 - 5.
Mardini S, Gohel A. Imaging of Odontogenic Infections. Radiol Clin North Am. 2018 Jan; 56(1): 31 - 44
Bali R, Sharma P, Ghanghas P, Gupta N, Tiwari JD, Singh A, Sapra N, Goyal D. To Compare the Efficacy of C-Reactive Protein and Total Leucocyte Count as Markers for Monitoring the Course of Odontogenic Space Infections. J Maxillofac Oral Surg. 2017 Sep; 16(3): 322 - 7.
Kumari S, Mohanty S, Sharma P, Dabas J, Kohli S, Diana C. Is the routine practice of antibiotic prescription and microbial culture and antibiotic sensitivity testing justified in primary maxillofacial space infection patients? A prospective, randomized clinical study. J Craniomaxillofac Surg. 2018; 46(3): 446 - 52. Epub 2017 Dec 20.
Lewis MA, MacFarlane TW, McGowan DA. A microbiological and clinical review of the acute dentoalveolar abscess. Br J Oral Maxillofac Surg. 1990; 28: 359 - 66.
Lucas VS, Gafan G, Dewhurst S, Roberts GJ. Prevalence, intensity and nature of bacteraemia after toothbrushing. J Dent. 2008 Jul; 36(7): 481 - 7.
Yamagata K, Onizawa K, Yanagawa T ym. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006; 38: 237 - 42.
Martins JR, Chagas OL Jr, Velasques BD, Bobrowski ÂN, Correa MB, Torriani MA. The Use of Antibiotics in Odontogenic Infections: What Is the Best Choice? A Systematic Review. J Oral Maxillofac Surg. 2017 Dec; 75(12): 2606.e1 - 2606.
Wang, Ahani, Pogrel. A five-year retrospective study of odontogenic maxillofacial infections in a large urban public hospital. Int J Oral Maxillofac Surg 2005; 34: 646 - 9.
Sato FR, Hajala FA, Freire Filho FW, Moreira RW, de Moraes M. Eight-year retrospective study of odontogenic origin infections in a postgraduation program on oral and maxillofacial surgery. J Oral Maxillofac Surg. 2009 May; 67(5): 1092 - 7.
Kinzer S, Pfeiffer J, Becker S, Ridder GJ. Severe deep neck space infections and mediastinitis of odontogenic origin: clinical relevance and implications for diagnosis and treatment. Acta Otolaryngol. 2009 Jan; 129(1): 62 - 70.
Henry M, Reader A, Beck M. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. J Endod. 2001; 27: 117 - 23.
Akbar I. Efficacy of Prophylactic use of Antibiotics to Avoid Flare up During Root Canal Treatment of Nonvital Teeth: A Randomized Clinical Trial. J Clin Diagn Res. 2015; 9: ZC08 - 1.
Keenan JV, Farman AG, Fedorowicz Z ym. Antibiotic use for irreversible pulpitis. Cochrane Database Syst Rev. 2005.
Robertson D1, Smith AJ. The microbiology of the acute dental abscess. J Med Microbiol. 2009 Feb; 58(Pt 2): 155 - 62.
Nordic theme 2018: Antimicrobial resistance.
Nordic Theme 2018: Prophylactic practices and guidelines in dentistry.
Corresponding author: Mataleena Parikka, Faculty of Medicine and Life Sciences, PO.B. 100, FI-33014 University of Tampere, Finland. E-mail: mataleena.parikka@uta.fi
This article has been submitted to peer reviews.
Parikka M, Norppa A, Välimaa H, Huttunen R, Järvinen A, Richardson R. Treatment of acute dental infections. Nor Tannlegeforen Tid. 2019; 129: 216 - 22
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Parikka M, Norppa A, Välimaa H, Huttunen R, Järvinen A, Richardson R. Treatment of acute dental infections. Nor Tannlegeforen Tid. 2019;130:0-0. doi:10.56373/2019-3-3