Acute dental pain I: pulpal and dentinal pain
The present article, a review aiming to update the reader on current knowledge on pulpal and dentinal pain, is the first in a series of articles on the theme "Pain and pain management".
The specialized anatomy of the pulp-dentin complex and the dense, predominantly nociceptive pulpal innervation from the trigeminal nerve explain the variety of pain sensations from this organ.
Brief, sharp pain is typical of A-fiber-mediated pain, while long-lasting, dull/aching pain indicates C-fiber involvement. A-fibers react to cold or mechanical stimuli, such as cold drinks or toothbrushing, while C-fibers are mainly activated by inflammatory mediators. Thus, lingering pain suggests presence of irreversible pulpal inflammation.
During pulpitis, structural changes of the pulpal nerves (sprouting) occur and neuropeptide release triggers an immune response; neurogenic inflammation. Pain sensations during pulpitis can range from hypersensitivity to thermal stimuli to severe throbbing, aching pain that can be referred and often difficult to localize making diagnosis a challenging situation for the clinician.
Surface biofilm amplifies hypersensitivity of exposed dentin surfaces because irritants reach the pulp through open dentin tubules, producing inflammation. Removing the biofilm reduces dentin hypersensitivity but supplemental treatment aiming to reduce dentin permeability, is often necessary. Caries removal and filling therapy is adequate during reversible pulpitis if the pulp has maintained its ability to distance itself from the bacterial assault by producing reparative dentin. However, endodontic therapy is necessary when pulpitis has reached an irreversible stage.
Pain localized to teeth is among the most frequently experienced orofacial pain complaints, with a prevalence of 12 % in the general population within a 6-month period (1). Tooth pain may be attributed to a variety of conditions, which may be acute or chronic in nature, local or systemic in origin, but is most frequently an indication of damage or disease in the tooth or its surrounding tissues. A good understanding of structures and mechanisms underlying the painful sensation is a prerequisite to pain management.
Innervation of the dental pulp and dentin
The dental pulp resides in a rigid capsule consisting of dentin and enamel. This creates a low-compliant environment that makes the pulp tissue unique (2, 3). The dental pulp is richly innervated mainly by axons from the trigeminal nerve, predominantly sensory in nature and mainly committed to pain perception (nociception). A smaller population of pulpal nerves are autonomic sympathetic fibers emanating from the superior cervical ganglion and associated with pulpal vasoconstriction (4).
Extremely strong pain - reaching the maximum intensity at any pain score - can be induced by activation of intradental nerves (5 - 7). Such intense pain responses can be explained by the dense (Figures 1 and 2) and predominantly nociceptive innervation of the pulp and dentin (6, 8). The transmission of the pain-inducing stimuli through dentin from its exposed surface is exceptionally effective and allows even very light stimuli, such as air blast and probing, to be intensified in a way that may induce tissue injury and subsequent nerve activation at the pulp-dentin border (5). Each tooth is innervated by about a thousand trigeminal axons (9 - 11), which may have branched before entering the apical foramen and may innervate more than one tooth. In the radicular pulp, the nerve fibers are bundled together, but once they reach the coronal pulp (8, 12, 13), they divide into smaller bundles. The axons then branch extensively and each may form 50 - 100 terminals in the peripheral pulp, forming a network under the odontoblast layer, known as the plexus of Raschkow. The density of nerve endings is especially high in the pulp horns, where as many as 50 % of the dentinal tubules are innervated. Many of the tubules contain multiple nerve terminals (8). There are approximately 20 000 - 30 000 nociceptive nerve endings/mm2 in the pulp-dentin border area in the most coronal pulp which accounts for the extremely high sensitivity of dentin.
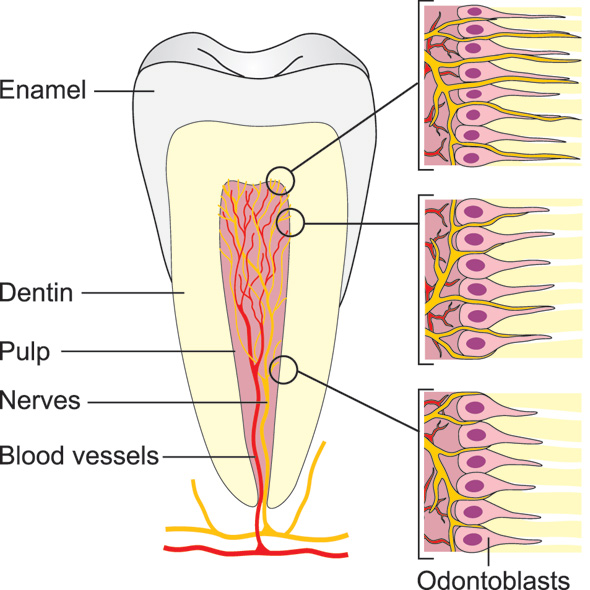
Figure 1. Schematic presentation of the intradental innervation. The nerve bundles enter the pulp via the apical foramen/foramina and branch extensively especially in the coronal pulp. The pulp-dentin border zone in the peripheral pulp (pulp tips) is the most densely innervated area, where the nerve endings also extend the longest distance (100 - 150 um) into the dentinal tubules.
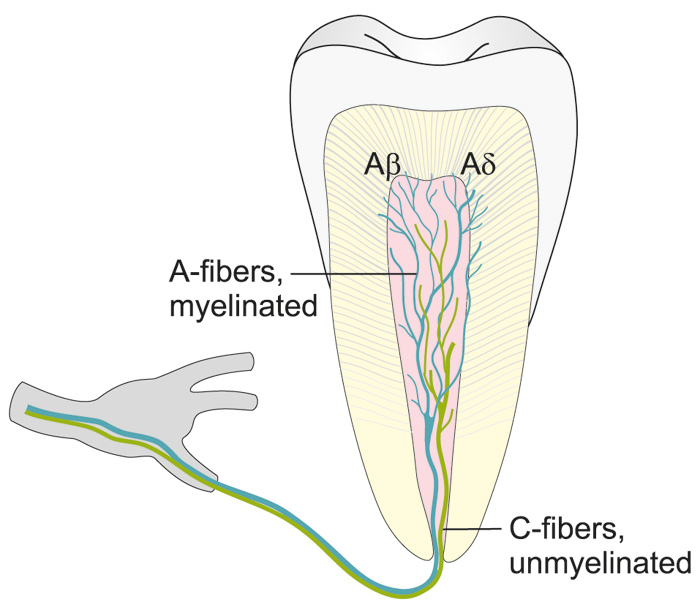
Figure 2. Illustration showing the distribution of intradental A- and C-fibers.
Unmyelinated C-fibers are located in the pulp proper, whereas myelinated A-fibers are extensively distributed in the pulp dentin border, penetrating the inner part of dentin.
Nerve fiber types: A- and C-fibers, their functional differences
There are both myelinated (20 - 25 %) and unmyelinated (75 - 80 %) afferent nerve fibers in the pulp (8, 12, 13). These two fiber groups differ greatly in their functional properties (6, 7, 14, 15). The myelinated fibers belong predominantly to the Ad- but a part of them to Ab-group and are fast conducting (from 3 up to 50 - 60 m/s (6, 7, 14, 15). The A-fiber endings are located in the peripheral pulp and inner dentin (Figs. 1, 2 and Table 1). They are responsible for dentin sensitivity, and their activation in healthy teeth results in sharp and usually short-lasting pain, not outlasting the stimulus (5 - 7, 16).
There are also a number of larger Ad-fibers (approximately 10 % that enter the pulp at the apex). These are not active in the healthy pulp but become active when inflammation is present. This is an example of `peripheral sensitization' when normally non-noxious nerve fibers are recruited to the pain system. All the sensory nerve fibers that enter the pulp branch and get narrower as they travel to the pulp cornua. Four times as many nerve fibers can be counted at the mid-crown level of the pulp than at the apical level. Myelinated nerve fibers commonly have non-myelinated terminals, making it difficult to differentiate the terminals of fast and slow fibers (13, 17).
The non-myelinated nerves are C-fibers having slow conduction velocities (0.5 - 2.5 m/s) and their terminals are located in the pulp proper. They are predominately sensory with a small population of sympathetics (10 %). The majority (70 %) of the axons entering the apex are C-fibers.
From the clinical point of view it is important to note that the sharp, short lasting, non-lingering, pain due to stimulation of exposed dentin can be evoked when the pulp is healthy or has some minor reversible injury and, thus, can successfully be managed without root canal treatment.
The C-fibers are polymodal and respond to several different noxious stimuli. In other sites they are activated by intense heat and cold and many inflammatory mediators such as histamine and bradykinin (7). In the pulp they are activated during inflammation, and increasingly so in its advanced stages (7). It seems that they may conduct the dull pain or ache in pulpal inflammation (5, 7).
Considering the response characteristics of the C-fibers it can be concluded that their activation, inducing dull aching pain, which is often long-lasting or lingering in nature, may suggest that the pulp is irreversibly damaged and might need root canal treatment.
In addition, nerve fibers release biologically active peptides, known as neuropeptides, which influence neural activity and functioning (18). Neuropeptides in the dental pulp are released from the nerve terminals of mainly A?- and C-fibers. There are numerous neuropeptides in the dental pulp which are commonly classified as sensory, sympathetic or parasympathetic according to the origin of nerve fibers. Sensory and sympathetic neuropeptides are synthesized in the trigeminal and superior cervical ganglion, respectively (19).
Sensory neuropeptides are e.g. calcitonin gene-related peptide (CGRP), substance P (SP) and neurokinin A. Neuropeptide Y (NPY) is co-released with noradrenaline from the sympathetic nerve terminals. The most abundant neuropeptide in the dental pulp is CGRP, followed by SP. CGRP is a vasodilator while SP increases capillary permeability. NPY is a vasoconstrictor and modulates the immune function (20). When injected in the blood stream in experimental studies, CGRP, SP and NKA produce vasodilation (21), whereas activation of pulpal nerves by electrical stimulation produces long-lasting vasodilation in the pulp due to release of CGRP (22 - 24).
Changes in the nerve function in inflammation, neurogenic inflammation, inflammatory mediators
Structural changes of nerve fibers occur in response to inflammation. Nerve fibers sprout or branch extensively (25, 26), thereby increasing the release of neuropeptides resulting in "neurogenic inflammation". CGRP and SP are increased at initial stages of pulpal inflammation, whereas NPY increases in chronic stages (27). Neuropeptides released from sensory neurons not only act on the vasculature, but also directly attract and activate innate immune cells (dendritic cells) and adaptive immune cells (T lymphocytes) (28, 29). Once immune cells are recruited to the site of inflammation, inflammatory mediators such as cytokines, histamine, bradykinin, prostaglandins, leukotrienes, and numerous other substances are released. Neural sprouting increases neuropeptide content and release, resulting in neurogenic inflammation (30, 31). Figure 3 schematically illustrates the 5 stages of changes to the dentin-pulp complex according to caries progression, possible symptoms and suggested treatment. Caries, even limited to the enamel layer may already have some minor effect on the dental pulp (5), e.g. in terms of neurogenic inflammation and onset of dentin sclerosis can occur, that corresponds with alteration along the odontoblast layer (32) (Stage 1). Sprouting of sensory neuropeptide containing nerve fibers occurs with deeper carious lesions (Stage 2 and 3) coinciding with hyper- and thermal sensitivity of a tooth (26, 31, 33). This sprouting is reversible and subsides to normal after caries arrestment or restoration. Irritation of the dental pulp due to caries leads to reparative dentin formation by odontoblasts. With the progression of caries (stage 4), localized microabscesses may form in the dental pulp with sprouting of nerve fibers. There is also increased release of neuropeptides (34).
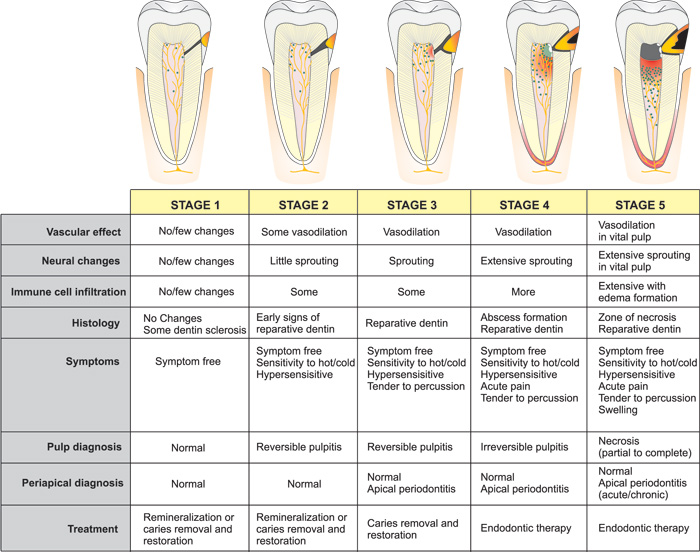
Figure 3. Schematic illustration of the 5 stages of caries progression from the enamel layer to pulp exposure with subsequent changes in the dental pulp (vascular, neural, immune, histological), possible symptoms and suggested treatment.
Increased release of sensory neuropeptides in the dental pulp causes vasodilation, leading to increased local tissue pressure and increased capillary permeability, causing plasma extravasation and edema formation. Due to the non-compliant nature of the dental pulp, clinically this can be felt as throbbing pain.
As the contaminated demineralized carious dentin reaches the dental pulp (stage 5), pulpal inflammation becomes extensive with partial necrosis combined with reparative dentin formation and vital inflamed pulp apically. Due to the loss of functional barrier against infection and limited capacity for healing in the coronal portion of pulp at this stage, necrosis progresses apically. Symptoms can be numerous and variable at this stage and when left untreated, infection and inflammation progresses, eventually leading to complete pulpal necrosis and apical periodontitis.
Mechanism of nerve activation in response to dentinal stimulation, dentin sensitivity
How stimuli are relayed from the peripheral dentin to the sensory terminals located in the region of the dentin-pulp border zone has been a subject of interest for many years. Evidence indicates that movement of fluid in the dentinal tubules is a crucial factor in dentinal pain. Pain-producing stimuli, such as heat, cold, air blasts, and probing with the tip of an explorer, have the ability to displace fluid in the tubules (35, 36). This is referred to as the hydrodynamic mechanism of dentin sensitivity.
Hydrodynamic theory
The hydrodynamic theory suggests that dentinal pain associated with stimulation of a sensitive tooth ultimately involves mechanotransduction. Recently, classical mechanotransducers have been recognized on pulpal afferents, providing a mechanistic support to this theory (37). Thus, fluid movement in the dentinal tubules is translated into electric signals by activation of mechanosensitive ion channels located in the axon terminals. Using single-fiber recording techniques, a positive correlation was found between the degree of pressure change and the number of nerve impulses leaving the pulp (38 - 40). The outward fluid movement (negative pressure) produces a much stronger nerve response than inward movements (36, 40).
A short application of cold or heat to the outer surface of dentin can evoke pain that is not dependent on temperature changes in the pulp (38, 41). The response to thermal stimulation is rapid, although the thermal conductivity of dentin is relatively low. Heat expands the fluid within the tubules, causing the fluid to flow towards the pulp, whereas cold causes the fluid to contract, producing an outward flow.
It is principally the A-fibers that are activated by a rapid displacement of the tubular contents (Table 1 and Figure 1) (42). C-fibers, however, may be activated by heat (above 43° C). The polymodal C-fiber nociceptors contain numerous receptors which respond to different types of stimuli (43, 44). Particularly, a receptor termed the "transient receptor potential, subtype vanilloid 1" or TRPV1 is expressed, and responds to heat above 43° C, certain inflammatory mediators, and acid (pH <6) (45). Eugenol activates and ultimately desensitizes TRPV1, and this may explain the anodyne action of zinc oxide eugenol temporary restorations (46).
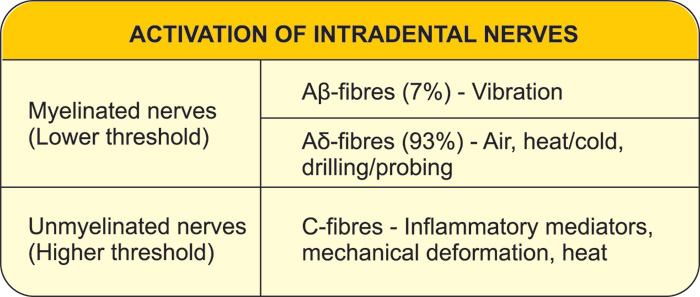
Table 1. Stimuli capable of activating the intradental nerves
It has also been shown that pain-producing stimuli are more readily transmitted from the dentin surface when the exposed tubule apertures are open and the fluid within the tubules is free to flow outward. For example, acid treatment of exposed dentin to remove the smear layer opens the tubule orifices and makes the dentin much more responsive to stimuli such as air blasts and probing.
The hydrodynamic theory is also applicable to explain hypersensitive dentin. It has been questioned whether exposed dentin is simply sensitive or becomes truly hypersensitive. However, evidence indicates that new sodium channels, capable of activating nerves, are expressed in nerve tissue exposed to inflammation. An increase in the density of sodium channels or their sensitivity may therefor contribute to dentinal hypersensitivity. Hypersensitivity typically occurs in the cervical area where the dentin is exposed because the protective enamel/cement was not formed or is worn out or etched away (Figs. 4 and 5). The odontoblasts and/or pulp cells respond by forming intratubular deposits or eventually tertiary dentin is laid down. This results in narrowing or closing of the dentinal tubuli. Deposition of tertiary dentin leads to decreased conductivity compared to the primary and secondary dentin. In addition, deposition of tertiary dentin without involvement of primary odontoblast cells over the pulpal ends of the exposed tubules may also reduce the sensitivity, as reparative dentin is less innervated by sensory nerve fibers. Some hypersensitive dentin, however, does not spontaneously desensitize, indicating either an ongoing inflammatory change or mechanical changes in the patency of dentinal tubules.
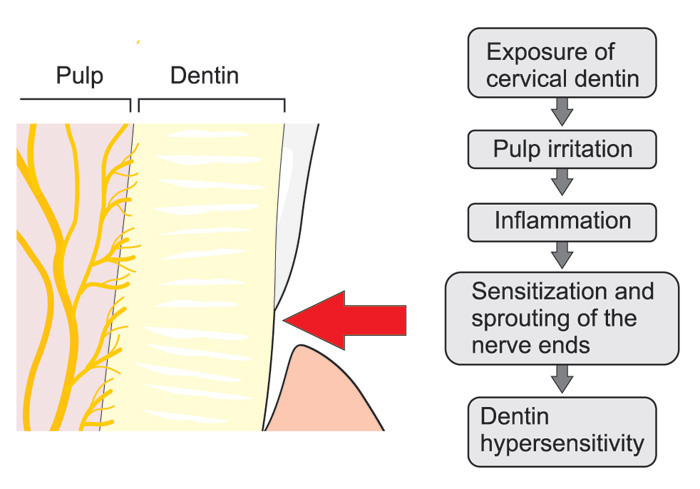
Figure 4. Possible neurogenic mechanisms playing a role in the development of cervical dentin sensitivity: After gingival recession external irritants may induce local inflammation in the pulp-dentin border and result in sprouting of the nerve endings and, consequently, more extensive innervation of the tissue compared to healthy teeth, which may increase dentin sensitivity due to the increased release of the neuropeptides together with many other inflammatory mediators, which may sensitize the nociceptive nerve endings.
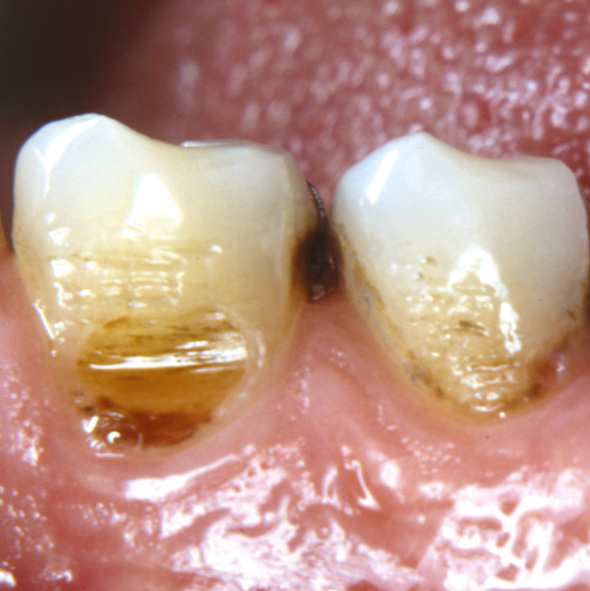
Figure 5. A wear facet is present at the buccal cervical surface of a lower canine. Due to pain the hygiene procedures were avoided. Eventually a carious lesion has started to progress at the gingival border.
Dentin hypersensitivity, development, prevention and treatment
The prevalence of individuals claiming to have dentin hypersensitivity has been reported to between 3 - 57 % and most frequently in patients between 20 and 40 years (47).
Bacteria and dentin hypersensitivity
A wear facet or non-carious cervical lesion may be very painful, and consequently the patient may avoid daily use of this particular tooth and oral hygiene procedures. This may in turn develop into even more severe pain. In cases where a biofilm develops, the bacteria and their metabolites penetrate the dentin, resulting in local inflammatory changes in the pulp, including neurogenic inflammation as described above. Due to the pain, the person may tend to leave the site undisturbed. This may have two clinical effects; firstly, an altered sensitivity of the nerves, which become more reactive, including the sequence of sprouting and secondly, there may be onset of caries progression (Figure 5). Taken together, the bacteria may play a role in severe dentin hypersensitivity, where only improved professional cleaning of the cervical area may lead to significant and permanent pain relief (48).
Iatrogenic development of hypersensitivity
During excavation the clinician may overextend the cavity preparation hereby exposing sound dentin (Figure 6), where the permeability of the dentinal tubules is higher than in subjacent carious dentin. This scenario may be accompanied with suboptimal cooling and dehydration of the dentin. Consequently, the patient may experience severe dentin hypersensitivity following excavation and restoration.
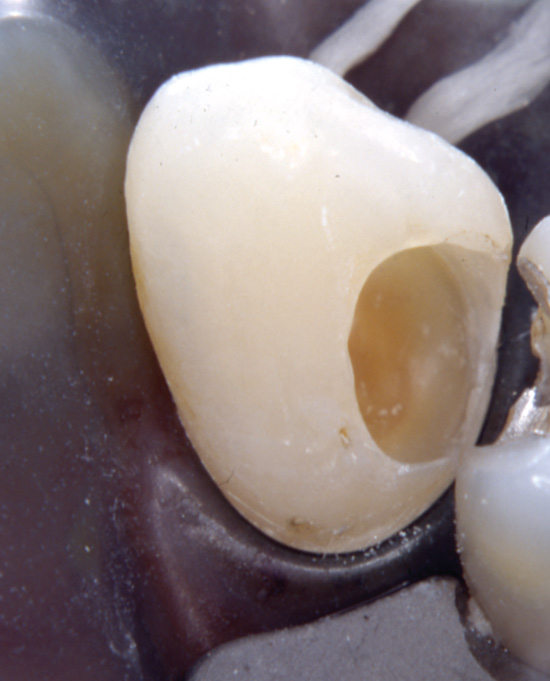
Figure 6. During excavation of caries an extensive peripheral excavation procedure may lead to a severe dentine hypersensitivity following restoration.
The role of pulpal inflammation in dentin sensitivity
Pulpal A- and C-fibers can be sensitized by many external irritants, which can induce an inflammatory response in the pulp tissue. Sprouting of the nociceptive nerve terminals takes place in response to inflammation and may widen the receptive fields of the nerve fibers (43, 49), which may result in increased overlap of the receptive fields (= the area where a single neuron can be activated, when stimulated). Thus, stimulation of a small spot e.g. in dentin may result in activation of a much greater number of pulpal nociceptors and, consequently, increased sensitivity compared to a non-inflamed tooth (Figure 7). Moreover, inflammation and the consequent sprouting of the axons may result in more extensive innervation in pulp and pulp-dentin areas which are normally sparsely innervated in healthy teeth. This may be one mechanism playing a significant role in increased cervical sensitivity (Figure 4). Also, fillings with open margins can induce pulpal inflammation, affecting the sensitivity of dentin in other parts of the pulp. Open dentinal tubules next to such a filling may allow the diffusion and penetration of external irritants into the pulp, resulting in inflammation, activation and also sprouting of the nerve endings in the pulp-dentin complex (Figure 8). In fact, it may well be that inflammation of some degree could in general play a role in dentin hypersensitivity.
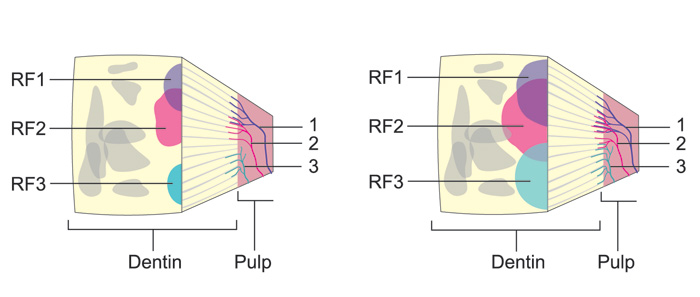
Figure 7. Schematic presentation of the receptive fields of single intradental nerve fibers in a block of dentin of a A: healthy and B: an inflamed tooth. Three nerve fibers with their nerve endings in the pulp-dentin border are indicated by numbers (1 - 3). They are colored (blue, red and brown) and the same colors are used to show their receptive fields on the exposed dentin surface. The receptive fields (RF) of the fibers are considerably wider and more extensively overlapping in the B: inflamed compared to the A: healthy tooth. Consequently, irritation of a standard area in dentin results in activation of considerably higher number of nerve fibers and higher sensitivity in the inflamed tooth compared to the healthy one.
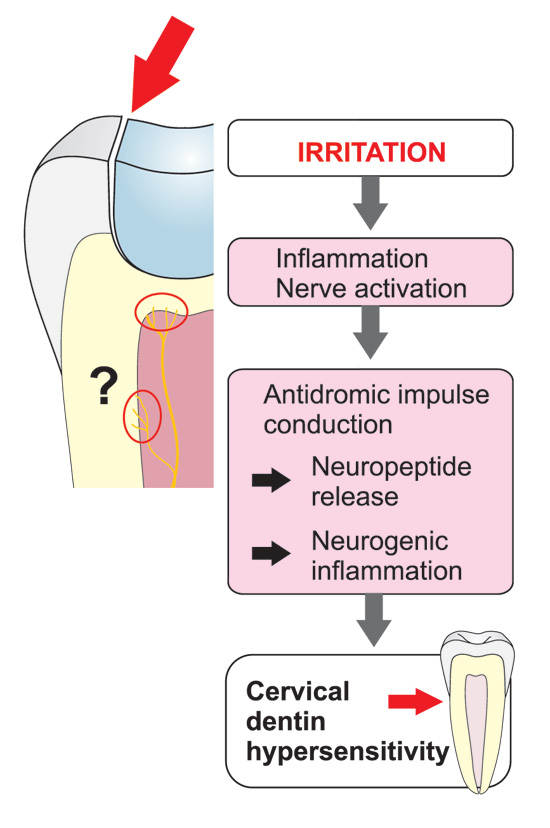
Figure 8. Even considerably remote leaky fillings may increase dentin sensitivity due to neurogenic inflammatory effects with branching of the intradental axons in other areas of the pulp and pulp-dentin complex. The neurogenic inflammation and related release of the neuropeptides induces activation of other inflammatory mediators as well. The induced inflammation increases the sensitivity of the pulpal nociceptors and, consequently, the sensitivity of the pulp and dentin. Accordingly, restoring the old, even remote fillings may sometimes be necessary for the treatment of dentin hypersensitivity.
In addition, dental pulp seems to contain a considerable number of so called "silent" or "sleeping" nociceptors that cannot be activated in healthy, but only in inflamed teeth (43). Electrophysiological experiments indicate that approximately 40 % of the nociceptive afferents can be activated in healthy teeth, whereas the proportion will increase to 60 % when the pulp is inflamed. Considering the total number of the intradental afferents (approximately 1000) in each tooth, such an increase in number of nociceptors is significant regarding the dental pain sensitivity.
Silent and "hot tooth«
It seems that the activation of pulpal nociceptors can vary to a great extent (5, 43, 50). In many cases acute pulpitis can be extremely painful. However, most often pulpal inflammation may proceed to total pulp necrosis with minor symptoms or with no symptoms at all (23, 50, 51) (Figure 3). This is puzzling considering the rich nociceptive innervation of the pulp. Such a variation in the symptoms can also be a serious diagnostic problem from the clinical point of view. A number of local mediators may be involved in the prevention of the nerve activation (43, 51). Those include e.g. local opioids, somatostatin, noradrenalin and nitric oxide (43, 52 - 54). These mediators are also important for regulation of the intensity of pulpal inflammation. The inhibition of nociceptor activity results in reduced release of the neuropeptides and other inflammatory mediators and also attenuation or even complete prevention of pain symptoms (43, 51). In addition to the local or peripheral sensitization and inhibition described above, mechanisms on brainstem level or higher in the central nervous system the complex nociceptive pathways may play an important role to regulate the pain (central sensitization/inhibition), like in all pain development and modulation (55) .
Clinical cases of pulpal and dentinal pain and their treatment
Treatment of dentin hypersensitivity
With reference to classical literature, the clinical impression and interpretation of dentin pain is something that will be triggered and provoked by well-defined external stimuli. In the following clinical scenarios, the accumulation of biofilm plays a huge role in leading to pain.
Scenario 1
The patient has not visited the dentist for years. The patient is completely unable to drink or have cold or hot fluids in the oral cavity due to pain. Clinically, the patient has a poor hygiene status and a high number of defective restorations. Accumulation of biomasses is noted during the clinical examination. Not only are the restorations suboptimal, but also larger parts of the teeth are broken down with exposed dentin. This has created an ecosystem with biofilm formation on exposed dentin (Figure 9a, b). Following several visits with only professional biofilm removal (Figure 9c), the patient arrives with a marked decrease in the pain level. Of course, in the real life scenario the clinician would initiate both hygiene procedures and restorations, but the present case reflects the important impact of biomasses on exposed dentin and dentin hypersensitivity.
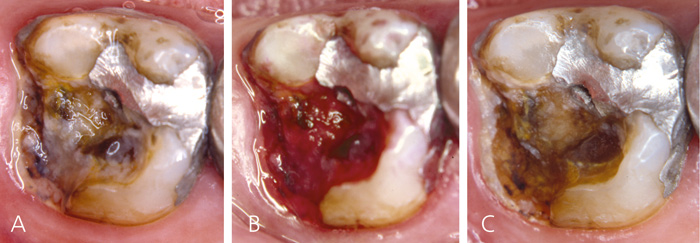
Figure 9. Patient presenting with severe dentin hypersensitivity and large cavities covered with biofilm. A: Before biofilm staining, B: After biofilm staining and C: after professional biofilm removal.
Scenario 2
If improved hygiene procedures has been introduced, but without pain relief the treatment plan should aim to reducing dentin permeability (Table 2). This can be achieved by either physically blocking the dentinal tubules or by depolarizing the nerves (56). A wide range of materials for desensitization have been sold but without universal success. Dentin hypersensitivity can be very difficult to control, indicating that the materials either have no permanent effect and/or that the inflammatory changes are so profound that a natural healing process is prevented. Several treatment modalities have shown an occluding or desensitizing effect in animals, but it has been difficult to demonstrate in the clinic (56). A problem with materials that are intended to block fluid flow in dentinal tubules, for example by precipitation of salt crystals, is that the precipitation may be washed out, is dissolved in an acid medium or is worn away so that the potential occluding effect is temporary.
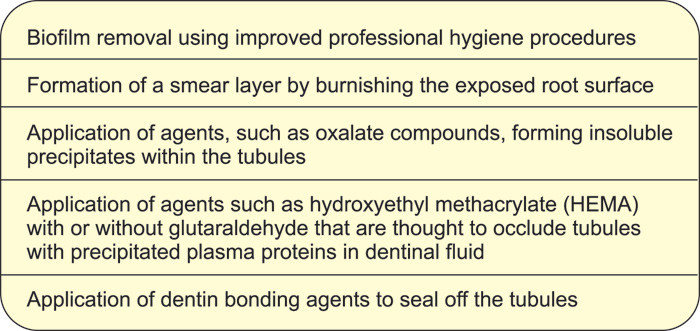
Table 2. Treatment modalities for treatment of hypersensitive dentin
There are many published studies on toothpastes with strontium salts and potassium salts (57). Several of these report that these toothpastes have some desensitizing effect, whereas others have not been able to demonstrate an effect. The design of these studies have been questioned and the effect is uncertain (58). Before conducting a costly and invasive treatment, a practical test could be to evaluate whether the individual patient feels improvement in symptoms using these toothpastes. A paste consisting of arginine and calcium carbonate, claimed to close the dentinal tubuli, has been introduced as a gentle treatment of a sensitive area. So far, only preliminary results are available from the manufacturer (59, 60).
If the above methods are ineffective the next step would be to seal the dentinal tubules with fluoride varnish, dentin primer and resin or with a resin restoration. In some cases it may end with endodontic treatment. As in other contexts, primary prevention is of course the optimal solution, by using a gentle brushing technique as well as a low intake of soft drinks with low pH as opposed to the attempt to treat the established hypersensitive area/lesion.
Conclusion
Diagnosis and management of dentinal pain is often a challenge to the clinician. The dental pulp is exceptionally richly innervated by nociceptive afferents, and pulpal and dentinal pain can cause patients considerable discomfort. Many factors are involved in the development and persistence of pain. Caries, iatrogenic damage, changes in dentin structure and permeability caused by erosion or tooth wear, aggregation of biofilm on unprotected dentin; all can lead to activation of nociceptive nerves which initiates local inflammatory changes in the pulp, and also triggers central changes in pain processing - both of which are complex and may be difficult to reverse.
References
Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993; 124(10): 115 - 21.
Heyeraas KJ, Kvinnsland I. Tissue pressure and blood flow in pulpal inflammation. Proc Finn Dent Soc. 1992; 88 Suppl 1: 393 - 401.
Kim S. Neurovascular interactions in the dental pulp in health and inflammation. Journal of endodontics. 1990; 16(2): 48 - 53.
Hildebrand C, Fried K, Tuisku F, Johansson CS. Teeth and tooth nerves. Prog Neurobiol. 1995; 45(3): 165 - 222.
Brännström M. Dentine and Pulp in Restorative Dentistry. Nacka, Sweden: Dental Therapeutics AB1981.
Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999; 10(1): 4 - 39.
Narhi MV. The characteristics of intradental sensory units and their responses to stimulation. Journal of dental research. 1985; 64 Spec No: 564 - 71.
Byers MR. Dental sensory receptors. Int Rev Neurobiol. 1984; 25: 39 - 94.
Johnsen D, Johns S. Quantitation of nerve fibres in the primary and permanent canine and incisor teeth in man. Arch Oral Biol. 1978; 23(9): 825 - 9.
Johnsen DC, Harshbarger J, Rymer HD. Quantitative assessment of neural development in human premolars. The Anatomical record. 1983; 205(4): 421 - 9.
Reader A, Foreman DW. An ultrastructural quantitative investigation of human intradental innervation. Journal of endodontics. 1981; 7(11): 493 - 9.
Hirvonen TJ. A quantitative electron-microscopic analysis of the axons at the apex of the canine tooth pulp in the dog. Acta Anat (Basel). 1987; 128(2): 134 - 9.
Holland GR, Robinson PP. The number and size of axons at the apex of the cat's canine tooth. The Anatomical record. 1983; 205(2): 215 - 22.
Jyvasjarvi E, Kniffki KD. Cold stimulation of teeth: a comparison between the responses of cat intradental A delta and C fibres and human sensation. The Journal of physiology. 1987; 391: 193 - 207.
Narhi M, Jyvasjarvi E, Virtanen A, Huopaniemi T, Ngassapa D, Hirvonen T. Role of intradental A- and C-type nerve fibres in dental pain mechanisms. Proc Finn Dent Soc. 1992; 88 Suppl 1: 507 - 16.
Narhi MV, Kontturi-Närhi V. Sensitivity and surface condition of dentin -- a SEM-replica study (abstract). Journal of dental research. 1994; 73: 122.
Beasley WL, Holland GR. A quantitative analysis of the innervation of the pulp of the cat's canine tooth. J Comp Neurol. 1978; 178(3): 487 - 94.
Maggi CCA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Progress in neurobiology. 1995; 45(1): 1 - 98.
Wakisaka S, Akai M. Immunohistochemical observation on neuropeptides around the blood vessel in feline dental pulp. Journal of endodontics. 1989; 15(9): 413 - 6.
Haug SR, Heyeraas KJ. Modulation of dental inflammation by the sympathetic nervous system. Journal of dental research. 2006; 85(6): 488 - 95.
Gazelius B, Edwall B, Olgart L, Lundberg JM, Hokfelt T, Fischer JA. Vasodilatory effects and coexistence of calcitonin gene-related peptide (CGRP) and substance P in sensory nerves of cat dental pulp. Acta Physiol Scand. 1987; 130(1): 33 - 40.
Gazelius B, Olgart L. Vasodilatation in the dental pulp produced by electrical stimulation of the inferior alveolar nerve in the cat. Acta Physiol Scand. 1980; 108(2): 181 - 6.
Olgart LM, Edwall B, Gazelius B. Neurogenic mediators in control of pulpal blood flow. Journal of endodontics. 1989; 15(9): 409 - 12.
Tonder KH, Naess G. Nervous control of blood flow in the dental pulp in dogs. Acta Physiol Scand. 1978; 104(1): 13 - 23.
Kimberly CL, Byers MR. Inflammation of rat molar pulp and periodontium causes increased calcitonin gene-related peptide and axonal sprouting. The Anatomical record. 1988; 222(3): 289 - 300.
Taylor PE, Byers MR, Redd PE. Sprouting of CGRP nerve fibers in response to dentin injury in rat molars. Brain Res. 1988; 461(2): 371 - 6.
Haug SR, Heyeraas KJ. Effects of sympathectomy on experimentally induced pulpal inflammation and periapical lesions in rats. Neuroscience. 2003; 120(3): 827 - 36.
Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997; 37(2 - 3): 133 - 52.
Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nature neuroscience. 2012; 15(8): 1063 - 7.
Haug SR, Berggreen E, Heyeraas KJ. The effect of unilateral sympathectomy and cavity preparation on peptidergic nerves and immune cells in rat dental pulp. Exp Neurol. 2001; 169(1): 182 - 90.
Taylor PE, Byers MR. An immunocytochemical study of the morphological reaction of nerves containing calcitonin gene-related peptide to microabscess formation and healing in rat molars. Arch Oral Biol. 1990; 35(8): 629 - 38.
Bjørndal L, Darvann T, Thylstrup A. A quantitative light microscopic study of the odontoblast and subodontoblastic reactions to active and arrested enamel caries without cavitation. Caries Res. 1998; 32(1): 59 - 69.
Khayat BG, Byers MR, Taylor PE, Mecifi K, Kimberly CL. Responses of nerve fibers to pulpal inflammation and periapical lesions in rat molars demonstrated by calcitonin gene-related peptide immunocytochemistry. Journal of endodontics. 1988; 14(12): 577 - 87.
Caviedes-Bucheli J, Lombana N, Azuero-Holguin MM, Munoz HR. Quantification of neuropeptides (calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and vasoactive intestinal polypeptide) expressed in healthy and inflamed human dental pulp. International endodontic journal. 2006; 39(5): 394 - 400.
Brännström M. The transmission and control of dentinal pain. In: Grossman LJ, editor. Mechanisms and control of pain. New York: Masson Publishing USA; 1979.
Matthews B, Vongsavan N. Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch Oral Biol. 1994; 39 Suppl: 87S-95S.
Hermanstyne TO, Markowitz K, Fan L, Gold MS. Mechanotransducers in rat pulpal afferents. Journal of dental research. 2008; 87(9): 834 - 8.
Narhi M, Jyvasjarvi E, Hirvonen T, Huopaniemi T. Activation of heat-sensitive nerve fibres in the dental pulp of the cat. Pain. 1982; 14(4): 317 - 26.
Vongsavan N, Matthews B. The permeability of cat dentine in vivo and in vitro. Arch Oral Biol. 1991; 36(9): 641 - 6.
Vongsavan N, Matthews B. The relationship between the discharge of intradental nerves and the rate of fluid flow through dentine in the cat. Arch Oral Biol. 2007; 52(7): 640 - 7.
Trowbridge HO, Franks M, Korostoff E, Emling R. Sensory response to thermal stimulation in human teeth. Journal of endodontics. 1980; 6(1): 405 - 12.
Narhi M. Activation of dental pulp nerves of the cat and the dog with hydrostatic pressure. Proc Finn Dent Soc. 1978; 74 Suppl 5 - 7: 1 - 63.
Narhi M, Yamamoto H, Ngassapa D. Function of intradental nociceptors in normal and inflamed teeth. In: Shimono M, Maeda T, Suda H, Takahashi K, editors. Dentin/pulp complex. Tokyo: Quintessence Publishing Co; 1996. p. 136.
Narhi M, Yamamoto H, Ngassapa D, Hirvonen T. The neurophysiological basis and the role of inflammatory reactions in dentine hypersensitivity. Arch Oral Biol. 1994; 39 Suppl: 23S-30S.
Chaudhary P, Martenson ME, Baumann TK. Vanilloid receptor expression and capsaicin excitation of rat dental primary afferent neurons. Journal of dental research. 2001; 80(6): 1518 - 23.
Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, et al. Activation of vanilloid receptor 1 (VR1) by eugenol. Journal of dental research. 2003; 82(10): 781 - 5.
West NX. Dentine hypersensitivity: preventive and therapeutic approaches to treatment. Periodontol 2000. 2008; 48: 31 - 41.
Hovgaard O. Dentin hypersensibilitet: fysiologi og behandling: Institut for Oral Anatomi og Tandsygdomslære, Århus Tandlægehøjskole; 1988.
Byers MR. Effects of inflammation on dental sensory nerves and vice versa. Proc Finn Dent Soc. 1992; 88 Suppl 1: 499 - 506.
Seltzer S, Bender IB, Ziontz M. The Dynamics of Pulp Inflammation: Correlations between Diagnostic Data and Actual Histologic Findings in the Pulp. Oral Surg Oral Med Oral Pathol. 1963; 16: 969 - 77.
Olgart LM. The role of local factors in dentin and pulp in intradental pain mechanisms. Journal of dental research. 1985; 64 Spec No: 572 - 8.
Fristad I, Berggreen E, Haug SR. Delta (delta) opioid receptors in small and medium-sized trigeminal neurons supporting the dental pulp of rats. Arch Oral Biol. 2006; 51(4): 273 - 81.
Olgart L. Neurogenic components of pulp inflammation. In: Shimono M, Maeda T, Suda H, Takahashi K, editors. Dentin/pulp complex. Quintessence Publishing Co1996. p. 169 - 75.
Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993; 76(1): 182 - 91.
Sessle BJ. The neurobiology of facial and dental pain: present knowledge, future directions. Journal of dental research. 1987; 66(5): 962 - 81.
Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008; 35(4): 300 - 15.
Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. 1963; 16: 846 - 71 contd.
Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006(3): CD001476.
Panagakos F, Schiff T, Guignon A. Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8 % arginine and calcium carbonate. American journal of dentistry. 2009; 22 Spec No A: 3A-7A.
Petrou I, Heu R, Stranick M, Lavender S, Zaidel L, Cummins D, et al. A breakthrough therapy for dentin hypersensitivity: how dental products containing 8 % arginine and calcium carbonate work to deliver effective relief of sensitive teeth. The Journal of clinical dentistry. 2009; 20(1): 23 - 31.
Corresponding author: Matti Nähri, e-mail: matti.narhi@uef.fi
This paper has been peer reviewed.
Närhi M, Bjørndal L, Pigg M, Fristad I, Haug SR. Acute dental pain I: pulpal and dentinal pain. Nor Tannlegeforen Tid. 2016; 126: 10-8.
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Närhi M, Bjørndal L, Pigg M, Fristad I, Rethnam
Haug S. Acute dental pain I: pulpal and dentinal pain. Nor Tannlegeforen Tid. 2016;126:10-8. doi:10.56373/2016-1-4