Human papillomaviruses and oral infections
Human papillomavirus (HPV) infects epithelial cells in the skin or mucous membrane, causing asymptomatic infection or benign lesions, squamous cell carcinoma or premalignant lesions. The natural history of HPV infection is best known in the female genital area. Research interest in oral infections has increased with the availability of commercial HPV vaccines. At the moment there is not enough information as to which HPV types infect the oral mucosa or on the natural history of HPV infections. The most common HPV genotype found in oral mucosa is HPV16. Asymptomatic oral HPV infections and infections with multiple types occur more frequently in immunocompromised patients; in addition, papillomas/condylomas caused by HPV take longer to heal. HAART, used for treating HIV, has been shown to increase the incidence of oral warts. The mechanism is largely unknown, but viruses can contribute to each other''s replication through reciprocal gene activation. Incidence rates of anal and cervical cancers caused by HPV have increased in HIV-infected patients, while there are only single case reports of oral cancer in these patients. Organ transplant patients also have more oral warts, which heal more slowly than those in healthy persons. It is likely that HPV vaccines also prevent oral HPV infections. However, the effect of the vaccine is affected by timing: prophylactic vaccine must be given before the first HPV infection. It is therefore likely that many immunocompromised patients have already been infected by HPV. Therapeutic HPV vaccines for treating cancer are still under development.
Papillomaviruses are small DNA viruses (8000 base pairs) that cause skin or mucosal hyperplasia at the site of infection. Papillomaviruses are species-specific, which is why human papillomavirus (HPV) affects only humans. Other species have their own specific papillomaviruses. The HPV genome contains 6 early (E) and 2 late (L) genes and a long control region (LCR) to which host cell proteins and the E2 protein of the virus bind (figure 1). Of the HPV genes, L1 is the one that shows the least variation in base order between different HPV types, which is why this gene is used as target gene in DNA-based HPV diagnostics. HPV classification is based on variability of nucleic acids in the L1 gene. HPV vaccines are also based on L1 protein which can self assemble into virus-like particles (VPL) when expressed as eukaryotic recombinant protein. Different types of HPV have been assigned numbers (e.g. HPV1, HPV6, HPV16 etc.) in the order in which they were identified, i.e. once their entire nucleic acid sequence was determined. At the moment, the number of HPV types that have been identified is 150. Based on their behaviour, the viruses can be divided into high- or low-risk HPV types, or based on the site of infection, into mucosal or skin types. Papillomaviruses were not accepted into the international virus classification until 2004. The majority of human papillomaviruses belong to the alpha group, which contains 15 different HPV species. Species 9 includes the most important cancer associated HPV-type, HPV16, and the other HPV types between 60-70% identity with it. Species 7 contains HPV18 and other HPV types close to it, while species 10 includes the most important benign types, HPV 6 and HPV 11. Of the viruses that infect oral mucosa, 15 represent high-risk and 12 low-risk HPV types (1).
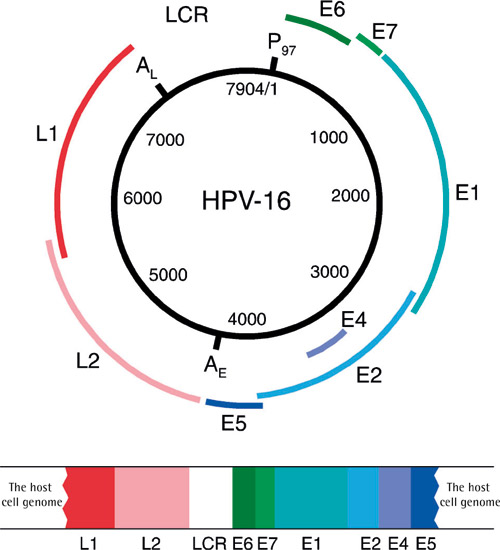
Figure 1. HPV16 genome and its binding to the host cell. (Published previously in Tandläkartidningen, 2006; 2: 41.)
Life cycle and cell transformation of the virus
According to the current view, HPV is unable to infect healthy skin or mucosa. Infection is possible only if the mucous membrane is wounded. The target cell of the virus is the basal epithelial cell. Possible receptors of HPV that enable the virus to enter the cell are alpha-6 integrin, laminin-332 and heparan sulfate proteoglycan. The E1 and E2 proteins of the virus appear first in the cell nucleus (figure 2). In the early phase of the virus life cycle, E6 and E7 proteins increase cell division and DNA synthesis and infere cell cycling, which enables significant replication of the virus genome (2). Replication and maturation of the virus are dependent on epithelial differentiation but the mechanism is not yet known. Therefore the virus cannot be cultured; as a result, viral diagnostics is based on microscopic determination of virus-induced cell changes or the detection of viral DNA or RNA in samples.
Viral particles are produced only in the surface layers of the epithelium. Upon exfoliation, they are released into their surroundings. The majority of HPV infections are transient and resolve spontaneously. If the infection becomes chronic, the risk of virus-induced cell changes increases. If this happens, HPV may become part of the host cell''s genome, leading to disruption of the E2 gene resulting in upregulation of E6 and E7 oncogenes (figure 2). Continuous production of these proteins provides a growth advantage to the infected cells. As the cell divides, its genome becomes more unstable, allowing mutations to accumulate. As a side effect of the infection, HPV infected cells become inmortal. Depending on host response, e.g. lack of immunological response, cells can gradually transform into cancer cells; as a result of cell division, this will gradually lead to tumour formation. E6 and E7 proteins bind many key host cell proteins; for example, E6 binds to p53 (so-called guardian of the genome), causing it to be degraded, while E7 binds to retinoblastoma protein (pRb), which allows uncontrolled cell division and bypassing of major checkpoints in cell division.
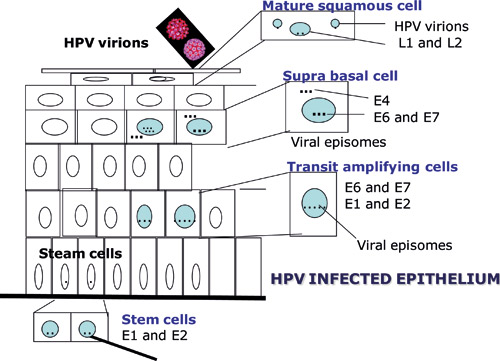
Figure 2. Schematic representation of the manifestation of HPV proteins during epithelial differentiation. (Published previously in Tandläkartidningen, 2006; 2: 44.)
Oral mucosa and HPV
Non-immunosuppressed patients
HPV infection in the oral mucosa may be asymptomatic, or manifest itself as benign lesions, precancerous lesions or even squamous cell carcinoma, depending on virus type (table 1).
Location |
Prevalence of HPV |
References |
|---|---|---|
Asymptomatic oral mucosa |
4.5 - 12.0 % |
6, 7, 15, 16 |
Lichen planus |
23.4 - 27.8 % |
6, 8 |
Leukoplakia, no dysplasia |
22.2 - 38.1 % |
6, 7 |
Dysplasia |
52.2 % |
6 |
Oral cancer |
23.5 - 46.5 % |
6, 7, 17, 18 |
Asymptomatic oral mucosa, HIV+ |
7.0 - 25.5 % |
16, 19, 20, 21 |
Asymptomatic oral mucosa, organ transplant patients |
20.0 % |
21 |
Asymptomatic infection. Asymptomatic oral HPV infection occurs in about 3 - 20 % of adults. The wide range may be explained by differences in sampling techniques and methods of sample handling, and especially by the methods used for HPV testing. HPV16 is the most common cause of asymptomatic oral infection. According to current knowledge, genital HPV infection does not increase the risk of oral infection (3). The natural, course of oral HPV infection is largely unknown, whereas that of genital HPV infection has been widely studied (4, 5). Genital HPV infections resolve in 6 - 12 months. HPV16 is also the most common HPV type in the genital area. A possible reason for this may be that epithelial cells can most easily be infected by this particular type of virus. HPV16 infection also takes the longest to clear; this has been thought to be due to the ability of the virus to prevent an immunological response. Deficiency of cell-mediated immunity is one of the most likely reasons for chronic infection.
Benign warts. Both warts (verruca) caused by skin-type HPV infections and condylomas and papillomas, which are primarily caused by HPV types 6 and 11, can occur in the oral mucosa. HPV types 13 and 32 cause focal epithelial hyperplasia (FEH), which is manifested clinically as single or multiple whitish lesions that disappear when the mucosa is stretched. FEH is familial, and the HLA types predisposing the mucosa to HPV13/HPV32 infections have been identified. FEH is not associated with increased risk of cancer.
Oral potential malignant disorders. HPV is found in about 20 - 25 % of oral leukoplakias (6 - 8). It is estimated that it is 4 - 7 times more likely to that HPV will be found in leukoplakia than in corresponding control samples. About 0.2 % of the lichen planus lesions turn of oral lichen planus turn malignant each year. Most recent studies have shown that HPV is also found in oral lichen planus, but its significance for the aetiology or malignant progression of the disease is unclear. The likelihood of finding HPV in oral lichen samples is estimated to be 5-fold that of control samples. Among these lesions, HPV16 was the most common finding, but HPV6 and HPV11 were found as well.
Oral cancer
The association between oral cancer and HPV has been controversial for nearly 30 years, whereas it has been indisputably shown in recent years that some (even 60 %) oropharyngeal head and neck cancers are caused by HPV. The problem with studies on cancer of the head and neck has been insufficient recording of the location of oral cancer and/or sampling. In 2010, the 5th World Workshop on Oral Medicine chose meta-analysis of HPV and oral cancer as one of its themes (6). The zero hypothesis was that HPV apparently plays no role in oral cancer. The selection criteria for the studies were strict; all in all, only 39 studies were accepted for this meta-analysis. Based on these studies, the material comprised 1,885 cases of oral cancer and 2248 control samples. HPV was found in 33.7 % of all cancer samples, compared with only 12 % of the control samples. Occurrence of HPV in the oral cancer samples was 3.98 times more likely than in control samples (95 % CI: 2.6 - 6.0). This supports the results of earlier meta-analyses of earlier results, where controls did not have to be included in the same study (7, 8). HPV16 was the predominant type, occurring in 68 % of oral cancers. Low-risk HPV types, particularly HPV6 and HPV11, are also found in oral cancers; accordingly, they are not necessarily low-risk types in the head and neck area. Patients with HPV-related head and neck cancer are younger, smoke less and have better prognosis than «classic» oral cancer patients, whose aetiology is associated with long-term alcohol use and heavy smoking (9). We do not yet know whether this is also true for oral cancer. However, due to differences in prognosis and even treatment between HPV-positive and HPV-negative cancers, HPV screening is about to become part of routine diagnostics.
HPV and immune deficiency
HPV and HIV.
It has long been known that HIV infection is associated with an elevated risk of genital and anal cancer and their precursors (10), whereas no corresponding association has been found for oral cancer (10). The introduction of antiretroviral treatments (HAART) has clearly reduced the occurrence of lesions caused by secondary infections in the oral mucosa (candidiasis, hairy leukoplakia, Kaposi''s sarcoma) seen in connection with symptomatic HIV, while oral warts have become more common and their treatment has become difficult due to relapses (12). Incidence of genital cancer in both men and women have also increased; initially this was believed to be a result of HAART treatment. Recent studies show that certain HIV proteins can activate HPV genes, and possibly also vice versa, which promotes the replication of both viruses. It has previously been shown that infection of the mouth with the herpes virus also facilitates oral HIV infection. It is unclear at the moment whether HPV and HIV also share the same mechanism.
Asymptomatic oral HPV infection. In persons infected with HIV, genital HPV infection increases the risk of oral HPV infection. It is also interesting that simultaneous infection with several HPV genotypes is more common among HIV-infected patients than among healthy persons. Among HIV-positive persons, smoking increased the risk of oral HPV infection becoming chronic. In addition, in connection with HAART treatments women have a higher risk of chronic oral HPV than men do (13).
Oral warts and HIV. In persons infected with HIV, oral warts are caused by skin-type HPVs and other mucosal HPV genotypes, in addition to HPV 6 and HPV 11. The cause of butchers'' warts, HPV7, is also a common cause of oral warts in HIV-infected patients. Oral infections caused by HPV32 are significantly more common among those infected with HIV than in healthy controls, especially when immune deficiency progresses and the viral load of HIV in the blood increases. The reason for this selection of HPV32 in particular is not known. Nor are the clinical changes or histology caused by different HPV types typical. For example, HPV32 may be found in lesions that histologically resemble warts, which may also simultaneously contain 3 - 5 different HPV genotypes.
Oral cancer, HPV and HIV
Cervical cancer is included in the definition of AIDS in HIV-infected persons. Incidencerates of anal cancer, in particular, have increased among both HIV-infected women and men (14), whereas few data are available on oral cancer and HIV. However, it has been predicted that the occurrence of HPV-induced oral cancer will increase among HIV-infected persons even as their survival improves, thanks to more efficient treatment.
Oral HPV and organ transplants
Organ transplant-related immunodeficiency increases the occurrence of HPV-induced warts and cancers. The majority of studies deal with the genital area. Apart from individual case reports, little is known about the prevalence of oral HPV infection in organ transplant patients. However, based on studies on persons infected with HIV, there is reason to suppose that occurrence of asymptomatic HPV in this patient group will increase and that some of them will get recurring warts that are difficult to treat as well as precancerous lesions and cancers.
Oral HPV and patients with rheumatoid arthritis
TNF-alpha antibodies have been shown to be an effective targeted treatment in patients with rheumatoid arthritis. However, some patients have experienced acutisation of other infections as a side effect caused by the treatment. At the moment it is not known whether the treatment in question may, for example, activate a latent oral HPV infection or delay spontaneous healing of warts.
Can HPV infection be prevented by vaccination?
There are currently two prophylactic HPV vaccines available: the bivalent (HPV16, HPV18) vaccine Cervarix® (GSK) and the quadrivalent (HPV6, 11, 16, 18) vaccine Gardasil® (Merck). These vaccines have been licensed worldwide. At the moment, the vaccines have been approved for prevention of cervical cancer and its precursors (Cervarix®); in addition, Gardasil has been approved for prevention of vulvar and vaginal cancer and their precursors as well as local adenocarcinoma. Gardasil is also intended for the prevention of genital warts (condyloma), as they are caused by HPV6 and 11. Gardasil is also currently being studied in men and seems to be effective in the prevention of both penis and anal cancer and their precursors. Studies have shown that both vaccines are highly effective in preventing precancerous lesions that occur in the genital area. The vaccine is effective in HIV-infected persons if they have not been infected with HPV prior to vaccination. Interesting new information has recently become available with regard to the healing of genital warts in immunocompromised patients with a delay of about 2 years after HPV vaccination. It is thus possible that the vaccines might also have a therapeutic effect, particularly in these patient groups where the mechanism is totally unknown.
HPV has a very stable genome, which is why it is likely that these vaccines are also effective against all infections caused by HPV16/18, regardless of the site of infection. Gardasil in different sites of the body a vaccine targeting four different types of HPV, might therefore prevent oral infection caused by HPV6, 11, 16 and 18. New HPV vaccines targeting nine different oncogenic HPV types are already undergoing clinical testing. However, the problem is that prophylactic vaccines should be given before the first infection. At the moment, the time of the first HPV infection cannot be known with certainty. Our studies of families have shown that infection may take place much earlier than has been supposed. HPV has even been detected in the placenta and umbilical blood. The significance of early infection is currently not known, e.g. whether it protects against new infections or whether it might even cause tolerance, so that virus proteins are not recognized as alien by the immunological system. Future research will shed light on these questions. About 70 - 80 % of the population need to be vaccinated for the effect of HPV to be visible on the population level. However, the current vaccination programmes in Canada, Australia and Scotland are already sufficiently extensive on the population level. Thus reliable information about the effect of the vaccines on the incidence of oral cancer may also first become available from these countries. Worldwide vaccination studies on the prevention of HPV infections of the head and neck have also been launched. Therapeutic vaccines, mainly targeted against HPV16 E6- and E7-proteins, are also undergoing clinical testing. Preliminary results have been promising, even though therapeutic vaccines have so far not been successful against any viruses.
Main points | |
|---|---|
· |
Deficiency in the human immune system increases the occurrence of oral lesions caused by HPV infection. |
· |
HIV infection and HAART treatment increase the occurrence of oral HPV infections and retard the healing of lesions caused by these infections. |
· |
Incidence rates of anal and cervical cancers caused by HPV have increased in HIV-infected patients, but so far there are only single case reports of oral cancer in these patients. |
· |
Organ transplant patients also have more oral warts and they heal more slowly than those in healthy persons. |
· |
It is likely that HPV vaccines also prevent oral HPV infections. However, the effect of the vaccine is affected by timing: prophylactic vaccine must be given before the first HPV infection. |
Literature
De Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004; 324: 17 - 27.
Doorbar J. Papillomavirus life cycle organization and biomarker selection. Dis Markers. 2007; 23: 297 - 313.
Xavier SD, Filho IB, de Carvalho JM, Castro TM, de Souza Framil VM, Syrjänen K. Prevalence of human papillomavirus (HPV) DNA in oral mucosa of men with anogenital HPV infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108: 732 - 7.
Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002; 2: 342 - 50.
Rintala M, Grenman S, Puranen M, Syrjänen S. Natural history of oral papillomavirus infections in spouses: a prospective Finnish HPV Family Study. J Clin Virol. 2006; 35: 89 - 94.
Syrjänen S, Lodi G, von Bultzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011; 17: 58 - 72.
Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982 - 1997. Oral Surg Oral Med Oral Pathol Endod. 2001; 91: 622 - 35.
Syrjänen K, Syrjänen S. Papillomavirus infections in human pathology. Wiley, London-Paris. 2000, pp. 1 - 615.
Gillison ML, D''Souza G, Westra W, Sugar E, Xiao W, Begum S et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Nat Cancer Inst. 2008; 100: 407 - 20.
Palefsky J. Human papillomavirus-related disease in people with HIV. Current Opin in HIV and AIDS. 2009; 4: 52 - 6.
Syrjänen S. Human papillomavirus infection and its association with HIV. J Dent Res. 2011; 23: 84 - 9.
Shiboski CH, Patton LL, Webster-Cyriaque JY, Greenspan D, Traboulsi RS, Ghannoum M, et al. Oral HIV/AIDS Research Alliance, Subcommittee of the AIDS Clinical Trial Group. The Oral HIV/AIDS Research Alliance: updated case definitions of oral disease endpoints. J Oral Pathol Med 2009; 38: 481 - 8.
Cameron JE, Hagensee ME. Oral HPV complications in HIV-infected patients. Curr HIV/AIDS Rep. 2008; 5: 126 - 31.
Kreuter A, Wieland U. Human papillomavirus-associated diseases in HIV-infected men who have sex with men. Current Opin in Infect Dis. 2009; 22: 109 - 14.
Kreimer AR, Bhatia RK, Messeguer AL, Gonzáles P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: A systematic review of the literature. Sex Transm Dis. 2010; 37: 386 - 91.
Fakhry C, D´Souza G, Sugar E, Weber K, Goshu E, Minkoff H, et al. Relationship between prevalent oral and cervical Human Papillomavirus infections in human immunodefiency virus-positive and -negative women. J Clin Microb. 2006; 44: 4479 - 85.
Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988 - 2007). Annals of Oncol. 2008; 19: 1681 - 90.
Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005; 14: 467 - 75.
Richer KL, van Rensburg EJ, van Heerden WF, Boy SC. Presence of Epstein-Barr virus, cytomegalovirus and human papillomavirus in normal oral mucosa of HIV-infected and renal transplant patients. Oral Dis. 2001; 7: 34 - 40.
Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004; 189: 686 - 98.
Ammatuna P, Campisi G, Giovannelli L, Giambelluca D, Alaimo C, Mancuso S, et al. Prevalence of Epstein-Barr virus and human papillomavirus in oral mucosa of HIV-infected patients. J Oral Pathol Med. 1992; 21: 164 - 70.
Corresponding author: Stina Syrjänen, Lemminkäisenkatu 2, FIN-20520 Turku. E-mail: stisyr@utu.fi
Artikkelen har gjennomgått ekstern faglig vurdering.
Syrjänen S, Rautava J, Willberg J. Human papillomaviruses and oral infections. Do HPV vaccines provide protection in patients with immune deficiency? Nor Tannlegeforen Tid. 2012; 122: 110-4.
Artikkelen er fagfellevurdert.
Artikkelen siteres som:
Syrjänen S, Rautava J, Willberg J. Human papillomaviruses and oral infections. Nor Tannlegeforen Tid. 2012;122:110-4. doi:10.56373/2012-2-12